- About Research & Innovation
- Advanced Cardiovascular Care
- Health Equity
- Inflammation
- Maternal and Fetal Medicine
- Musculoskeletal Care
- Neuroscience
- Opioid & Pain
- Research Centers
- Departments
- About the Office of Research
- Funding & Proposal Development
- Regulatory Review & Compliance
- Research Project Management
- Cores & Resources
- Education & Training
- Peter Arvan Lab
- Scott Soleimanpour Lab
- Antiphospholipid Syndrome Research Labs
- J. Michelle Kahlenberg Lab
- John Varga Lab (ScleroLab)
- Mulholland Lab
- Pasca di Magliano Lab
- Raghavendran Lab
- ALS Center of Excellence
- Institute for Heart & Brain Health
- Center for Basic & Translational Science
- Center for Bioethics and Social Sciences in Medicine
- Biomedical Research Core Facilities
- IT Route Map
- Clinical Research Route Map
- Commercialization Route Map
- Great Minds, Greater Discoveries
- Research Scouts
- Meet the ROMS Team
- ROMS Fellowship Application Information
- Working with a ROMS Fellow
- Pandemic Research Recovery
- Research Climate Council
- Support for Outstanding Research
- Research News Trivia
- Frequently Asked Questions (FAQ)
- Billing Calendar & Study Applications
- CRAO Frequently Asked Questions (FAQ)
- Pre-Onboarding Considerations
- Access Biospecimen
- Biospecimen Processing
- Biospecimen Inventory
- Regulatory & Governance
- Equipment & Instrumentation
- Central Biorepository Pricelist
- CBR Frequently Asked Questions (FAQ)
- Clinical Trials Support Units
- Clinical Research Coordinator Career Ladder
- Clinical Trials Management Systems: Accounts & Support
- Research Pharmacy
- Statistical Support for Large-Scale Clinical Trials
- MCRU Services
- MCRU Lab Study Team Guidelines
- MCRU Frequently Asked Questions (FAQ)
- Michigan Medicine Site Profile
- CTSO Frequently Asked Questions (FAQ)
- Self-Serve Data Tools
- Custom Data Request
- Data & Biospecimen Sharing
- Student Access to Patient Data
- FFMI fastPACE
- Frankel Cardiovascular Center Innovation Program
- Rogel Cancer Center Innovation Program
- Global Health Commercialization Competition
- Biomedical Innovation 101
- Certificate in Biomedical Innovation & Entrepreneurship
- Fast Forward Medical Innovation Fellowship Program
- Biotech Career Development Program
- Drug Discovery & Development Course
- Innovation Studio
- Oncology Drug Discovery & Development (3D) Workshop
- FFMI fastPACE Train-the-Trainer
- Intellectual Property in the Academic Setting
- Drug Discovery and Development Course
- Medical Device Regulation
- Idea to Impact Webinar Series
- 6 Tips for Customer Discovery
- Interacting with Industry
- Ask the Experts: A Biomedical Innovation Forum
- Business Development
- Michigan Biomedical Venture Fund
- R01 Boot Camp
- Facility & Resource Profile Templates
- BMRC Bridging Support Program for Biomedical Research
- Medical School Limited Submissions
- Budgeting & Costs
- NIH Specifics, Tips & Tricks
- Grant Routing Deadlines
- Proposal Review Checklists
- Department Contacts
- Authorized Signers
- Research Data Analytics
- Grant Processing Advisory Committee (GPAC)
- Post-Award Advisory Committee (PAAC)
- Grants Frequently Asked Questions (FAQ)
- Board Nominations Sought
- Informed Consent & Assent Templates
- IRBMED Seminar Series
- Information for IRB Members
- Information for Study Subjects
- SignNow Electronic Signature Service
- IRBMED Frequently Asked Questions (FAQ)
- IRBMED Contacts & Roster

A path for career progression.
In 2022, Michigan Medicine implemented a career ladder for all staff currently performing clinical research coordinator work.
Research is one of the primary missions of Michigan Medicine. Recognizing the talent and expertise of these individuals to our mission as well as professionalizing the role was one of the top priorities identified by department chairs as part of their strategy to transform the clinical trials enterprise at Michigan Medicine. In recent years, the number of individuals performing important work to administer research has increased and the market titles assigned to these individuals have not been consistent across the organization. This series of job titles within clinical research will be a more consistent way to classify employees based on their responsibilities, competencies, and experience. This series also provides employees with a clear path for career progression within Michigan Medicine.
The following are market titles in this career ladder:
- Clinical Research Assistant ( 103921 )
- Clinical Research Technician ( 103922 )
- Clinical Research Coordinator Associate ( 103923 )
- Clinical Research Coordinator Intermediate ( 103924 )
- Clinical Research Coordinator Senior ( 103925 )
- Clinical Research Coordinator Lead ( 103926 )
- Clinical Research Project Manager ( 103927 )
View the Michigan Medicine policy outlining the required use of the Clinical Research Coordinator (CRC) career ladder.
A summary of the job descriptions is available in U-M Career Path Navigator, as linked above, and in Michigan Medicine Total Rewards. U-M supervisors and staff can review the detailed job descriptions (requires login) and summary job grid for the most appropriate title based on position responsibilities and an individual’s competencies. After determining the appropriate title, download the respective template (requires login) for job postings.
To ensure consistency of use across the organization, the Senior, Lead or Project Manager titles will require review by CRC Governance Board prior to job posting, candidate offer, and reclassifications.
As we professionalize the clinical research coordinator role at Michigan Medicine, professional certification is a key component of career progression in our competency- and training-based framework. ACRP CCRC , SoCRA CCRP , or equivalent certification is required for any positions at CRC – Associate or higher with new employees permitted six months from start date to acquire.
The expense of certification will be the responsibility of the hiring individual (e.g., PI) or central unit (e.g., Department, Division, Center, Institute, Program, etc.). The hiring individual or central unit will be responsible for the costs associated with clinical research coordinators maintaining their certification, such as continuing education and recertification fees. The source of funds for these expenses may include institutional funds, gift funds, or sponsored projects, as appropriate and proportional to the activity of the individual.
For more information on certification reimbursements, click here .
The CRC Career Ladder competency domains:
- Scientific Concepts
- Ethical and Participant Safety Concerns
- Investigational Products Development and Regulations
- Clinical Study Operations
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
These competencies were developed by the Joint Task Force for Clinical Trial Competency , an international team of investigators, educators, and clinical research professionals. This framework defines the knowledge, skills, and attitudes necessary for conducting safe, ethical, and high-quality clinical research.
CRC SharePoint Resource Library
Requirements to Use of the Clinical Research Coordinator Career Ladder Policy
Clinical Research Coordinator Certification Requirements Policy
Clinical Research Coordinator Reimbursements for Certification
The Clinical Trials Support Office is a unit of the Medical School Office of Research, where our mission is to foster an environment of innovation and efficiency that serves the Michigan Medicine research community and supports biomedical science from insight to impact.
We transform lives through bold discovery, compassionate care and innovative education.
- Diversity, Equity & Inclusion
- News & Stories
- Find a Doctor
- Conditions & Treatments
- Patient & Visitor Guide
- Patient Portal
- Clinical Trials
- Research Labs
- Cores and Resources
- Programs & Admissions
- Our Community
- Departments, Centers & Offices
- About the Medical School

Global Footer Secondary Navigation
Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)

Thomas Boothby, MS, CCRP CRA II, Boston Scientific
Abstract : Research coordinators may transition to clinical research associates/monitors during their careers. This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process. A roadmap in the form of a checklist is provided to help make the transition from research coordinator to clinical research associate (CRA) a smooth one.
Disclosure: The author has a relevant financial relationship with respect to this article with Boston Scientific, where he is employed as a monitoring CRA.
Introduction
A research coordinator is a person at the clinical research site who is involved in the daily tasks of enrollment, data entry, and all other aspects of clinical trials at the site level. A clinical research associate (CRA), or monitor, is the individual who visits clinical research sites to review their medical records and do the standard monitoring visits. Before the author was a CRA, he was a research coordinator for fourteen years. This article describes how the author made the transition from clinical research coordinator to CRA/clinical research monitor and includes some suggestions for those looking to make a similar career change.
When to Transition from Research Coordinator to CRA
While people naturally want to progress their careers as fast as possible, it is important to only make thetransition from research coordinator to CRA when the time is right. The grass is not always greener on the other side of the work fence.
The author knew that he was ready to make the transition from research coordinator to CRA because he felt that he had mastered all the tasks of a research coordinator. His job became stagnant, and he was looking for something better. Fatigue in the current work environment is another reason for why individuals may be looking to make this transition. Of all members of the clinical research team, research coordinators have the most difficult job. In the author’s opinion, they are often overworked and underpaid, and their contributions to the overall study are sometimes overlooked. Other reasons to make the transition from research coordinator to CRA include potential career progression and the opportunity to try something new. Some individuals may find that the travel component that goes along with being a monitor is a positive as well.
There are five stages of change according to a behavioral change model: pre-contemplation, contemplation, determination, action, and maintenance. In the pre-contemplation phase, people are not thinking about transitioning yet or may have obstacles in their daily lives that are preventing them from exploring new opportunities. When people are becoming serious about change, they are in the determination or action phases. During these phases, research coordinators who want to transition to CRAs might apply for new positions or become certified clinical research professionals (SOCRA CCRP®) as they try to gain new skills for the job market. When considering a transition from research coordinator to CRA, it is important to identify one’s place in the behavioral change model.
Qualifications and Background of CRAs
When the author was applying for CRA positions in 2015, he always saw a requirement for at least two years of experience as a monitor. This requirement is often a barrier to those looking to make this career transition. In 2010, ClinicalTrials.gov listed more than 100,000 clinical studies. By 2019, that number has increased to more than 300,000 clinical studies. The clinical research market has exploded over the last decade. More people are needed to monitor and to run clinical studies now than ever before. While some companies are less likely to require two years of monitoring experience now due to a depleted pool of candidates, these same companies may be more open to supplemental forms of experience such as certifications, course work, and on-the-job experience.
Thus, this is a great time to act on the decision to transition from research coordinator to CRA. From 2014 to 2024, the United States Bureau of Labor Statistics estimates that CRA positions will increase 14% annually. This increase in the job market, coupled with the high level of CRA turnover, could lead to a very strong job market in the future. At Boston Scientific, turnover among CRAs is fairly low due to the strong structure and principles. Many CRAs within Boston Scientific have been with the company for 10 to 20 years or longer.
Table 1 highlights the typical background of CRAs. Most CRAs are current or former nurses who have experience as a research coordinator or a research assistant. Many universities now offer bachelor’s, master’s, and certificate programs in clinical research as another form of training for these research related roles. In Michigan, where the author is from, Eastern Michigan University has a two-year master’s degree program in clinical research. Like the author, CRAs can often be a former research coordinator.
When the author was transitioning from research coordinator to CRA, he got his foot in the door by working closely with a monitor who still works for Boston Scientific. Relationships between research coordinators and CRAs can be contentious due to the nature of monitoring. Research coordinators should treat monitors and sponsor staff well and with respect, and they should treat monitoring visits as a learning opportunity and not a criticism of the coordinator’s work. These relationships do not need to be contentious. A good working relationship with a clinical research site’s CRAs can serve as a potential audition for a monitoring position.
CRAs typically have a clinical research certification, either SOCRA’s certified clinical research professional (CCRP®) or the Association of Clinical Research Professionals-Certified Professional (ACRP-CP). Some companies provide tuition reimbursement for programs and certifications such as these as a way of employee enhancement. Research coordinators can participate in enrichment programs such as these and obtain certifications to help boost their resume and become more marketable to CROs and sponsors. When researching these programs, individuals must do their due diligence to ensure that the program or certification is offered by a legitimate organization and is accredited. Hiring managers know where to find the gold standards in clinical research programs and certifications, and those that do not fit this standard can even be viewed as a negative on ones resume.
The author is a SOCRA CCRP®, Certified Clinical Research Professional, which is an excellent indicator of knowledge for a monitoring position. The test includes knowledge of the regulations and the role of the monitor. There are also some CRO-development programs such as SOCRA’s Clinical Research Monitoring Conference and one-year certificate programs such as the Harvard Medical School global clinical scholar’s research training program.
Networking through the clinical research site’s CRAs and professional forums and groups such as SOCRA is a great way to find CRA positions and interact with other research professionals. At conferences, CROs often have booths in the exhibit hall where research coordinators can meet CRO staff, learn more about opportunities, and leave their resume with CRO staff.
A Typical Day in the Life of a CRA
The life of a CRA has its positives and negatives (Table 2). There are many things that the author wishes he knew before he became a monitor. The author works from home a great deal of the time. If he is not on the road visiting a clinical research site, he is working at home either preparing for a visit, writing follow-up visit letters, or performing other administrative work. Visit preparation and follow up is a crucial part of the home office work. CRAs have very strict compliance guidelines for completing monitoring visits and monitoring reports in a timely manner. Since recently becoming a lead CRA, the author has also been doing a great deal of administrative and compliance work with more of a global view of a clinical trial.
Some clinical research organizations (CROs) and sponsors have onsite monitors who can do remote visits and activities. Whether visits are onsite or remote, monitors are constantly in contact with clinical research sites to follow up on action items from monitoring visits or to answer protocol specific questions the site coordinators may have.
At most companies, about 60-80% of the monitor’s time is spent traveling to sites. The author currently covers all of Michigan, and he has covered other areas, including Wisconsin, New York, Pennsylvania, and Ohio. CRAs are often away for several days at a time depending on the current workload. This can be difficult on families and personal relationships. While the author travels extensively, there are some times when he travels more than others. Sometimes he does back-to-back visits and may be gone for several days at a time. After that, he may be home for several days. The extensive travel required of CRAs is a key consideration when exploring this career transition.
Being a CRA takes a great deal of self-discipline. Monitoring offers a flexible work arrangement, so monitors can work later in the day or take time off during the normal workday. However, if the CRA does not accomplish what he/she should accomplish, this will be glaringly obvious. Management and co-workers will immediately know if the CRA does not show up to meetings or has difficulty answering questions about his/her monitoring activities or their monitor role in general.
Starting a Monitoring Job
Boston Scientific has a rigorous onboarding process comprised of four to six months of training. After the author was hired as a CRA, he spent months learning the work instructions and going out on preceptor visits. In the beginning, the new CRA observes a senior CRA. Over time, the new CRA does more of the monitoring. By the end of the training, the new CRA is doing the monitoring visit, and the senior CRA is observing and making suggestions to the new hire on how the new hire can improve.
There are various levels of monitors at Boston Scientific: CRA I (for new hires), CRA II, and senior CRA. More experienced CRAs often mentor new CRAs. It is extremely helpful to find CRAs who can serve as mentors and answer questions.
CROs and sponsors have many systems that CRAs must learn. At Boston Scientific, these systems include electronic data capture, clinical trial management, auxiliary programs to help remote employees, and cloud-based filing systems. Being a CRA might be very difficult for people who are resistant to change or have difficulty with technology.
There are several types of monitoring (Table 3). The author would be considered a traditional CRA or monitor. By this, he does traditional onsite monitoring via annual or semi-annual visits to clinical research sites based upon the study’s monitoring plan. At smaller organizations, monitors may travel more often or may have an expanded territory to cover. It is important to ask how much travel is involved and how many monitors are on the team during the interview process. If a company has fewer monitors, more travel will be involved.
Many Boston Scientific protocols require annual monitoring visits. The author visits his clinical research sites at minimum once a year but generally 2-3 times per year. Some of the more difficult sites, high enrollers, and those that are more likely to be inspected by the U.S. Food and Drug Administration are monitored more often. Many sites are participating in more than one Boston Scientific study. For example, the author monitors a site in New York that is conducting several studies. He will monitor two studies during one visit. This saves him time and travel and saves the company money by reducing travel costs. Boston Scientific also uses a risk-based monitoring strategy.
In-house regulatory CRAs at Boston Scientific, called trial management CRAs, interact with the sites on regulatory matters, study startup, and study closure. They work primarily by email and lean on traditional CRAs such as the monitor to be the face of the company with the research coordinators and help ensure that tasks are completed on time. Many hospitals also run their own clinical studies and may have in-house monitors.
Boston Scientific does use remote monitoring in certain studies and circumstances. Remote monitoring takes a great deal of work and technological experience at both the sponsor and site level. It involves a great deal of scanning and correspondence by the research coordinators, which can take a lot of their time and resources.
Sponsor CRAs generally deal with one indication, while CRO CRAs can work on studies for different indications or therapies. In one month, for example, CRO CRAs may be doing four indications at four sites for four sponsors. This requires understanding a great deal of information and being able to use different systems. Good organization is key when working as a CRA, whether for a sponsor or a CRO.
Recently, the author progressed from a CRA II to a senior CRA. As a senior CRA, the author has a larger leadership role and is expected to participate more in training and mentoring other CRAs. Boston Scientific has some centralized monitoring that will look at certain metrics and internal documents to guide monitors in their daily monitoring activities. Monitors are closely linked to the trial managers who actually run the studies. They also deal with safety and data managers as well as their CRA manager and the director of operations. Boston Scientific recently created an associate clinical trial manager position as a way to slowly transition some staff members into clinical trial managers, and the author is also transitioning into this role.
One common drawback about this transition process from research coordinator to CRA is that a CRA is one step removed from patient care. Working directly with patients as a research coordinator is something that the author misses. It is important to remember that CRAs help protect patients who are participating in clinical studies at more of an indirect level. This ideology helps prevent burnout, especially when monitors are swamped with the many reports that are necessary as part of the monitoring process.
Checklist for Transitioning from Research Coordinator to CRA
Table 4 has a checklist for determining whether one is ready to make the transition from research coordinator to CRA. Prior to applying for positions, the research coordinator must consider his/her stage in the behavior change model. Unless the research coordinator is ready to transition to a CRA position, he/she should not do it. Becoming a CRA can be difficult without two to five years of research experience in medical devices, pharma, or academia in some capacity. A research coordinator who wants to transition to a CRA should work closely with current CRAs who can provide mentoring and networking opportunities as well as exploring other networking avenues such as SOCRA and ACRP forums, LinkedIn, and also attending the annual events or local events put on by these organizations.
It is important for research coordinators to bolster their resumes by completing supplemental training or certifications. Resumes should be up-to-date and attractive to potential employers. This means including details about accomplishments along with basic information such as job titles and education.
The research coordinator must also consider the travel demands of a CRA position, the types of monitoring to pursue, and his/her stage in the behavior change model. Travel is a major part of a CRA position and should be a focal point of your conversation with a hiring representative. Finally, the types of monitoring including central monitoring, remote monitoring, and regional monitoring should be considered.
Monitoring is a great job. It allows a lot of freedom. However, CRAs also have a great deal of responsibility. CRAs must be driven, willing to put in the time, and have the necessary work ethic while maintaining vigilance and holding others accountable for good clinical practices.
Typical Background of a CRA
- Nursing degree with a clinical research background
- Bachelor’s or master’s degree in clinical research
- Former/current research coordinator
- Clinical experience (medical assistant, registered nurse, or nurse practitioner)
- Clinical research certified ( SOCRA CCRP ® or ACRP-CP)
- Research experience/background
- Science/academic research background
The Life of a CRA
- This requires being self-motivated and driven
- Sometimes performing a combination of onsite and remote monitoring
- Email, etc.
- At times, CRAs are gone for several days at a time depending on current workload
- Visit preparation and follow-up is a crucial part of work at home
Types of Monitoring
- Annual or semi-annual visits based upon the monitoring plan
- Risk-based monitoring/central monitoring
- Remote monitoring
- In-house CRAs and regulatory CRAs
- Sponsor CRA/monitor
- CRO CRA/monitor
Checklist for Transitioning from a Clinical Research Coordinator
to a Monitoring CRA (Clinical Research Associate)
- 2-5 years of research experience as a research coordinator or research assistant
- Able to work with current CRAs as part of a mentorship or network with CRAs
- Completion of supplemental training or certifications to support career goals and bolster resume
- Explore networking avenues
- Up-to-date resume that is attractive to potential employers
- Able to meet travel demands of a CRA position
- Consideration of types of monitoring to pursue
- Stage in the behavior change model
7 thoughts on “Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)”
Your articles are always helpful and I always get something new to learn from them.
Research Update Organization
you are always giving something new. thank you for that.
Very clear and helpful article! The tables listing the different types of monitoring roles and the items to consider whether this is right transition are a great summary, too.
- Pingback: An overview of a career in clinical research: What jobs are available in this field? - Rebiz Zield
This is a very clear and collective article. The tables are the best helpful tips and resources for anyone interested for career advancement in clinical research. I really appreciate the author’s time and effort to sharing this article.
- Pingback: Career Growth Opportunities in Clinical Research
- Pingback: Introduction to Clinical Research: What Every Student Should Know - Drug Safety and Pharmacovigilance Course
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
33RD ANNUAL CONFERENCE
ACHIEVING EXCELLENCE IN CLINICAL RESEARCH :
FORGING STRATEGIC COLLABORATIONS
September 27 to 29
COUNTDOWN TO ANNUAL CONFERENCE
Join us for expert-led sessions, interactive workshops, a peer-driven poster program, an engaging exhibit program and unparalleled networking opportunities!

News & information
Keep up with the latest from r&d partners, 10 clinical research career paths and progression opportunities, felicia rodriguez.
- October 30, 2023
Clinical research careers contribute to the development of safe and effective treatments and therapies for patients. The responsibilities may vary based on the organization, therapeutic area, and specific study requirements, but they all share the common goal of advancing medical science and improving healthcare outcomes.
Progressing in clinical research jobs involves a combination of experience, education, certifications, and networking. All of these are fairly essential for career growth, although the specific path and opportunities may vary depending on your interests, the organization you work for, and the area you specialize in.
In this guide, we explore some of the many careers in clinical research, from entering the profession to potential progression opportunities.
Clinical research career paths
Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities.
1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol adherence. They coordinate study visits, maintain documentation, and communicate with investigators.
2. Clinical research associate (CRA) Entry-level clinical research associate jobs involve monitoring clinical trial sites, verifying data, ensuring regulatory compliance and study protocols, and assessing the safety and wellbeing of study subjects.
3. Clinical trial manager Clinical trial managers oversee all operations of a clinical trial, from study initiation to close-out. They manage budgets, timelines, and teams of CRAs, ensuring that trials are executed successfully.
4. Clinical project manager Clinical project managers manage and oversee multiple trials within a program. They collaborate with cross-functional teams, manage resources, and ensure that each phase of a project aligns with organizational goals.
5. Regulatory affairs specialist Regulatory affairs specialists are largely responsible for the administrative side of compliance. They compile, submit, and maintain regulatory documents for approval. You’ll have to stay informed on changing regulations and liaise with regulatory agencies, ensuring that all policies are adhered to.
6. Data manager Data managers manage clinical trial data, overseeing data collection, cleaning, and database management. They ensure data quality and see to it that all standards are followed, working closely with biostatisticians.
7. Clinical research scientist Clinical research scientists design study protocols, collect and analyze data, and interpret results. They also write study reports and publish findings in scientific journals.
8. Medical monitor Medical monitors oversee patient and subject safety during the trial, review adverse events, and make recommendations for study adjustments or halts based on medical knowledge.
9. Clinical quality assurance auditor Auditors conduct regular inspections and audits to adhere to regulations and quality standards. They identify non-compliance issues and recommend corrective actions.
10. Clinical research consultant Consultants provide expert guidance on various aspects of clinical research, including study design, regulatory strategies, and data analysis. They work independently or with organizations to solve complex problems.
Clinical research progression opportunities
To advance in clinical research careers, you can further your education by pursuing advanced degrees. These might include a Master’s in Clinical Research or an MBA. You could also obtain certifications relevant to your role, like the Clinical Research Professional (CCRP) or Project Management Professional (PMP).
Networking can also help you to get ahead by building rewarding relationships, connecting with peers and mentors in the industry and learning from more senior professionals. This could involve attending conferences, joining professional organizations, and asking to shadow leaders.
Here’s more detail on clinical research progression, and areas the above ten roles can move into.
1. Senior CRC or Clinical Research Associate (CRA) Clinical research coordinators (CRCs) can progress to a senior CRC or clinical research associate (CRA). Responsibilities include more independent study management, training junior coordinators, and handling complex trials.
2. Senior CRA or clinical trial manager Clinical research associates (CRAs) can progress to a senior CRA or clinical trial manager position. The duties of senior clinical research associate jobs are focused on starting to mentor junior CRAs. Clinical trial managers take on even more responsibility by overseeing an entire team of CRAs.
3. Clinical project manager or senior clinical trial manager Clinical trial managers can progress to clinical project manager roles, which involve managing the entire project portfolio. Senior clinical trial managers then demonstrate their capabilities by handling significantly more complex trials.
4. Director of clinical operations Clinical project managers can look to become directors of clinical operations. Directors play a pivotal role in overseeing the management and execution of clinical research programs within an organization, including teams of project managers and CRAs.
5. Regulatory affairs manager or director Over time, regulatory affairs specialists might be able to take up a manager position, which would involve handling larger portfolios of products. Eventually, you could become a director, overseeing an entire regulatory department.
6. Senior data manager or clinical data scientist Data managers often move into senior roles, which means managing larger datasets. You could then set your sights on becoming a data scientist, specializing in data analysis.
7. Senior research scientist or director of clinical research With experience, clinical research scientists can aim to become senior scientists. You would take on more significant research projects, possibly with the goal of becoming a director. This would mean running an entire research department.
8. Chief medical officer (CMO) Medical monitors can progress to chief medical officers (CMOs), who are responsible for all medical aspects of clinical research within an organization.
9. Senior auditor or quality assurance manager Later in their careers, clinical quality assurance auditors might become senior auditors, overseeing an audit team. You could then take up a quality assurance manager position, managing the entire quality assurance program.
10. Clinical research consultant As a consultant, your options for progression are slightly different. Rather than looking to move into a different role, the goal is usually to build a larger client base and gain expertise in specific therapeutic areas. Your earnings and reputation can then grow as you become an expert in the field.
Progressing your career with R&D Partners clinical research staffing agency
R&D Partners are dedicated to helping you excel in the rewarding field of clinical research. As experienced clinical research job recruiters, we understand that a rewarding career in this industry can shape the future of healthcare, making a positive impact on people’s lives.
Whether you’re looking for entry-level clinical research associate jobs or senior leadership roles, our goal is to provide you with insights, strategies, and guidance to chart your path to success in this ever-evolving industry. As partners to many leading life science organizations on the east and west coast, we can bring you exclusive career opportunities not available anywhere else.
Contact our friendly team to discuss your career options, or browse our current opportunities in clinical research careers.
Our global FSP & strategic staffing firm specializes in Scientific, Clinical Research, and Engineering.
Explore our top job opportunities.
We offer life sciences niche functional service provider & strategic staffing solutions to help you reach your goals.
How to become a clinical research coordinator
Is becoming a clinical research coordinator right for me.
The first step to choosing a career is to make sure you are actually willing to commit to pursuing the career. You don’t want to waste your time doing something you don’t want to do. If you’re new here, you should read about:

Still unsure if becoming a clinical research coordinator is the right career path? Take the free CareerExplorer career test to find out if this career is right for you. Perhaps you are well-suited to become a clinical research coordinator or another similar career!
Described by our users as being “shockingly accurate”, you might discover careers you haven’t thought of before.
How to become a Clinical Research Coordinator
Becoming a clinical research coordinator involves a combination of education, relevant experience, and specific skills. Here is a guide on how to pursue a career as a CRC:
- Obtain a Relevant Bachelor's Degree: Most CRC positions require a bachelor's degree in a relevant field such as biology , chemistry , nursing , psychology , or healthcare administration . Some positions might prefer candidates with a degree in a specific area related to the type of clinical research they are involved in.
- Gain Clinical Experience: Having some clinical experience, such as working as a medical assistant, nurse, or laboratory technician, can be highly beneficial. This experience provides a foundation of medical knowledge and an understanding of healthcare practices, which are valuable in the field of clinical research.
- Pursue Additional Certifications (Optional): While not always mandatory, obtaining certifications related to clinical research can enhance your credentials. The Society of Clinical Research Associates (SoCRA) and the Association of Clinical Research Professionals (ACRP) offer certifications such as Certified Clinical Research Professional (CCRP) and Certified Clinical Research Coordinator (CCRC) respectively. These certifications demonstrate your expertise and commitment to the field (see below).
- Gain Entry-Level Experience: Entry-level positions in clinical research, such as research assistant or data entry clerk, can provide valuable experience and help you understand the intricacies of clinical trials. Networking within the clinical research community can also open doors to job opportunities.
- Pursue Advanced Education (Optional): Some CRCs choose to pursue master's degrees (such as Master of Public Health or Master of Clinical Research) to enhance their knowledge and career prospects. Advanced degrees can be particularly useful if you aspire to move into leadership or specialized roles within the clinical research field.
- Develop Relevant Skills: CRCs need a diverse skill set, including attention to detail, organizational skills, communication skills, and the ability to work in a team. Developing these skills, along with a good understanding of medical terminology and research methodologies, is crucial for success in this role.
- Apply for CRC Positions: Keep an eye on job listings from academic institutions, hospitals, research organizations, and pharmaceutical companies. Tailor your resume and cover letter to highlight your relevant experience and skills. Be prepared for interviews that might include questions about your understanding of clinical research protocols, ethical guidelines, and your ability to handle various responsibilities of a CRC.
- Continue Professional Development: Clinical research is a constantly evolving field. Stay updated with the latest developments, regulations, and technologies. Consider attending conferences, workshops, and training programs to enhance your knowledge and skills.
Certifications In the field of clinical research, certifications can enhance your credentials and demonstrate your expertise and commitment to prospective employers. Here are two widely recognized certifications:
- Certified Clinical Research Professional (CCRP) by the Society of Clinical Research Associates (SoCRA): SoCRA offers the Certified Clinical Research Professional (CCRP) certification, which is designed for individuals involved in the conduct and management of clinical trials. To be eligible for the CCRP exam, candidates typically need a combination of education and work experience in clinical research. The certification exam tests candidates on their knowledge of regulations, ethics, clinical trial conduct, and other essential topics. Obtaining CCRP certification demonstrates your expertise in clinical research practices and ethical standards. For more information about the CCRP certification, including eligibility requirements and exam details, you can visit the SoCRA website: SoCRA CCRP Certification.
- Certified Clinical Research Coordinator (CCRC) by the Association of Clinical Research Professionals (ACRP): ACRP offers the Certified Clinical Research Coordinator (CCRC) certification, which is aimed at professionals responsible for the coordination and administration of clinical trials. To be eligible for the CCRC exam, candidates need a combination of education and work experience in clinical research. The CCRC certification exam assesses candidates' knowledge of various aspects of clinical research, including protocol implementation, ethics, and participant safety. For more information about the CCRC certification, including eligibility requirements and exam details, you can visit the ACRP website: ACRP CCRC Certification.

CareerCatalyst
Explore Programs
All Programs
Certificates
Technical Bootcamps
Workforce Education
Artificial Intelligence
Leadership and Management
Technical skills
Professional skills
Sustainability and ESG
Microelectronics
- Skip to main content
- Report an accessibility problem
- Colleges and Schools

How to Become a Clinical Research Coordinator
Unlock the pathway to a rewarding career as a Clinical Research Coordinator with insights on duties, CCRC certification training options, and salary expectations.
In the rapidly advancing field of healthcare and medical research, Clinical Research Coordinators (CRCs) play a pivotal role. As the backbone of clinical trial operations, they not only ensure the seamless execution of studies but also bridge the gap between innovative medical discoveries and enhanced patient outcomes.
By meticulously managing research protocols and ensuring compliance with ethical standards, CRCs are instrumental in translating medical breakthroughs into tangible healthcare improvements. Their dedication to patient safety and advocacy further underscores their essential contribution to the future of medicine and patient care.
Here, we’ll explore what the role entails, career and education pathways and next steps for you to become a Clinical Research Coordinator .
What is a Clinical Research Coordinator?
In the world of medical research, a Clinical Research Coordinator (CRC) makes sure that research studies and clinical trials run smoothly and effectively. Imagine them as the glue that holds together everyone involved, from doctors and scientists to the participants in the studies.
At its core, a CRC ensures that research follows all the rules, ethical standards, and laws. They are the ones who oversee the day-to-day operations of a study, making sure everything happens according to the plan. Picture yourself as the captain of a ship—organize your crew (participants and healthcare professionals), navigate the course (protocol adherence), keep a detailed log (data management), and ensure your ship follows all maritime laws (regulatory compliance).
Primary Duties and Responsibilities:
A Clinical Research Coordinator (CRC) ensures every note is played perfectly in the symphony of clinical trials and research studies. Let's break down the key tasks that make up their daily score:
- Clinical Trial Coordination:
- Develop and implement study protocols.
- Coordinate all aspects of clinical trial activities.
- Collaborate with investigators, sponsors, and regulatory authorities.
- Participant Recruitment and Screening:
- Devise recruitment strategies.
- Screen and enroll eligible participants.
- Maintain accurate participant records.
- Document and Record Management:
- Maintain comprehensive study documentation.
- Ensure compliance with regulatory and ethical standards.
- Prepare and submit regulatory documents.
- Regulatory Compliance:
- Monitor and ensure adherence to regulations.
- Facilitate IRB submissions and address compliance issues.
- Safety and Supply Management:
- Implement safety protocols.
- Monitor and manage study-related materials and supplies.
- Cost Analysis and Budgeting:
- Conduct cost analysis and prepare budgets.
- Track expenditures and adhere to budget constraints.
- Training Programs:
- Design and deliver training for staff and participants.
- Ensure all team members are well-versed in protocols.
- Data Collection and Maintenance:
- Collect and maintain accurate study data.
- Implement quality control measures.
- Participant Point of Contact:
- Act as the main contact for participants.
- Address participant questions and concerns.
- Study Promotion:
- Promote the study through various channels.
- Collaborate on marketing and outreach efforts.
How Much Do Clinical Research Coordinators Get Paid?
Understanding the compensation landscape is essential as you consider a career as a Clinical Research Coordinator (CRC). Salaries for CRCs can vary based on several factors, providing a range of earning potential. On average, entry-level CRCs can expect a salary in the range of $45,000 to $60,000 per year. As you gain experience and expertise in the field, mid-level CRCs typically earn between $60,000 and $80,000 annually. Those with advanced skills and a wealth of experience, such as Senior Clinical Research Coordinators, can command salaries exceeding $90,000 per year.
It's important to note that the geographical location can significantly impact salaries. Metropolitan areas and regions with a high demand for clinical research professionals tend to offer higher compensation to attract and retain top talent. For instance, CRCs working in urban centers may earn salaries at the upper end of the pay scale compared to those in rural areas.
In addition to base salaries, many CRCs receive benefits such as health insurance, retirement plans, and bonuses tied to the success of clinical trials. Furthermore, opportunities for career advancement, continuing education, and professional development can enhance the overall compensation package.
Research average salaries in your desired location and industry to align your salary expectations with the prevailing market rates. Equip yourself with negotiation skills and insights into maximizing your earning potential as you embark on this fulfilling career path.
CCRC Certification Requirements:
The Certified Clinical Research Coordinator (CCRC) credential is your passport to credibility in the field. The certification, recognized globally, showcases your dedication to excellence in clinical research. Leverage CCRC preparation resources, including practice exams and guidance from seasoned professionals, to increase your chances of success in obtaining this prestigious certification.
Pathways into the CRC role and beyond
Embarking on a career as a Clinical Research Coordinator (CRC) involves a structured pathway to ensure you possess the necessary qualifications and skills . Here are the key steps to guide you on your journey:
Bachelor's Degree : Obtain a bachelor’s degree, preferably a Bachelor of Science in a relevant subject such as clinical research administration, health sciences, public health, microbiology, or a related field.
Work Experience : Gain practical experience in healthcare or clinical research. This can be achieved through internships, voluntary assignments, or entry-level roles in hospitals or research positions. This experience is crucial for developing the skills required in the field.
Master's Degree or Graduate Certificate : While a bachelor's degree is the minimum requirement, consider pursuing a master's degree or a graduate certificate to enhance your qualifications. Specialized subjects like clinical research, clinical administration, or clinical research management are advantageous. The flexibility of part-time or online study can accommodate your work commitments.
Certifications : Though not mandatory, obtaining a clinical research certification is recommended for career advancement. The Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SOCRA) offer certifications that validate your skills and competence. Options include:
- ACRP Certified Professional (ACRP-CP)
- Clinical Research Coordinator (CCRC)
- Clinical Research Associate (CCRA)
- Principal Investigator (CPI)
- ACRP Medical Device Professional Subspecialty (ACRP-MDP)
- ACRP Project Manager Subspecialty
The field of clinical research is dynamic, and continuous learning and adaptation are key to a successful career. By following these career pathway steps, you'll be well-prepared to navigate the complexities of clinical research coordination and contribute to groundbreaking advancements in healthcare.
Your Next Steps:
As you consider the exciting journey of becoming a Clinical Research Coordinator, explore CareerCatalyst’s Clinical Research Coordinator Program , designed to ensure that you are well-prepared to thrive in the future of clinical research.
Develop the skills to succeed at every career stage.
You’re one step closer to leaving your footprint on the world. Request information today and we will be in touch with you shortly.
- Art and Design
- Communication and Digital Media
- Computer Science and Technology
- Cybersecurity
- Engineering
- Entrepreneurship and Innovation
- Geographical Sciences and Urban Planning
- Health and Wellness
- Information Technology
- Law, Criminal Justice and Public Service
- Liberal Arts
- Project Management
- Social and Behavioral Sciences
- Sustainability
- No elements found. Consider changing the search query.
- List is empty.
By completing and submitting your information to ASU, you consent to:
- ASU using this information to contact you regarding information as you requested and send information about degree programs, scholarships, opportunities, events, and admission through email, direct mail, SMS/texting and digital platforms. You can unsubscribe at any time by clicking the unsubscribe link at the bottom of any email or by contacting [email protected] .
- The terms of ASU's Privacy Statement .
- If you are in the European Union or another country or state that has adopted the GDPR (General Data Protection Regulation) or similar privacy protection, please also read the ASU European Supplement to ASU's Privacy Statement .

Have questions? We’ve got the answers. Ask away!
Business hours Monday - Friday 7am - 6pm MST
- [email protected]
- 1-844-353-7856
Program Experience
ASU CareerCatalyst News

Clinical Research Career Path: A Complete Guide
Clinical research offers a rewarding career path in the field of medicine, combining scientific inquiry with the goal of improving patient care and developing new therapies. This comprehensive guide provides valuable information on the diverse opportunities, progression paths, and necessary qualifications in the clinical research industry.
Key Takeaways:
- Clinical research involves evaluating the safety and efficacy of medical treatments on human volunteers through rigorous scientific trials.
- Randomized Controlled Trials (RCTs) are considered the gold standard for assessing the effectiveness of medical interventions by minimizing bias.
- Clinical Research Organizations (CROs) play a critical role in overseeing and managing clinical trials for pharmaceutical and biotech companies.
- Clinical research career options include roles such as Biostatistician, Clinical Research Associate (CRA), Clinical Trial Manager, and more.
- Progression in clinical research careers can lead to positions like Clinical Director or Vice President, requiring further experience and expertise.
- Networking and obtaining certifications from associations like ACRP, SoCRA, CCRA, and ICR can enhance career prospects in clinical research.
- The average salaries for clinical research professionals vary by country, with Australia and India offering competitive compensation packages.
By following this complete guide, individuals aspiring to pursue a career in clinical research can gain valuable insights into the industry, understand various career paths, and make informed decisions about their professional development. Unlock the world of clinical research and contribute to advancements in medical knowledge and patient outcomes.
The Importance of Clinical Research Careers
Clinical research careers play a vital role in the development of safe and effective treatments for patients. These careers are crucial in advancing medical science and improving healthcare outcomes.
With the ever-increasing demand for new therapies and medications, clinical research professionals are at the forefront of innovation and discovery. They contribute to the development of breakthrough products and treatments that address a wide range of conditions, including heart disease, rheumatoid arthritis, Alzheimer’s, cancer, and even diseases like COVID-19.
Clinical research is responsible for the development of new medicines and vaccines that save lives and improve quality of life. It plays a vital role in combating antibiotic resistance, a pressing global health issue. Despite no new antibiotics being discovered since 1984, clinical research continues to explore new avenues and approaches to tackle this challenge.
Moreover, clinical research careers offer significant opportunities for development and advancement. Programs like the CRA Graduate Programme and the CRA Academy Programme provide structured pathways for new graduates and professionals with industry experience to excel in their careers.
For those interested in pursuing a career in clinical research, having a science degree, particularly in science, bio-medicine, nursing, or pharmacy, is highly valued. The dynamic and varied nature of clinical research involves activities such as setting up, monitoring, and closing clinical studies, traveling to different research areas, attending meetings, and collaborating with various stakeholders.
Career Opportunities and Salary
The clinical research industry offers diverse career opportunities with competitive salaries. According to statistics, the average salary for a Clinical Trial Assistant in 2020 was $63,000. Clinical Research Coordinators earned an average salary of $63,117, and Clinical Research Associates with one to two years of experience had an average salary of $72,358. With six years of experience, CRAs can earn up to $110,102 annually. Individual contractor CRAs even have the potential to earn up to $300,000 a year. Starting salaries in clinical research are considered relatively high, with ample room for progression towards high-paying positions.
Furthermore, the global market for clinical trials is projected to grow significantly, reaching $69.9 billion by 2027 and $84.43 billion by 2030. This growth indicates the increasing demand for clinical research professionals and the abundant opportunities for career development.
In addition to the competitive salaries, many companies in the industry offer attractive benefits such as private healthcare, dental care, and vision care. Job security in clinical research is robust due to ongoing pharmaceutical work, technological advancements, and the continuous need for new treatments.
In conclusion, clinical research careers are of utmost importance in the development of safe and effective treatments. These careers provide rewarding opportunities for professionals to make a positive impact on global healthcare while enjoying competitive salaries and extensive career progression.
Clinical Research Career Statistics
| Statistic | Value |
|---|---|
| Life sciences sector turnover in the UK in 2020 | £88.9 billion |
| Participants involved in clinical research studies supported by the National Institute for Health Research Network in 2019 | Over 870,000 |
| New studies supported by the National Institute for Health Research in 2019 | 2,194 |
| Global clinical trials market value in 2019 | $46.8 billion |
| Projected global market for clinical trials by 2027 | $69.9 billion |
| Average salary for a Clinical Trial Assistant in 2020 | $63,000 |
| Average salary for a Clinical Research Coordinator in 2020 | $63,117 |
| Average salary for a Clinical Research Associate with one to two years of experience | $72,358 |
Clinical Research Career Options
When considering a career in clinical research, there are various options available for professionals with different skills and interests. These roles play an essential role in ensuring the safety and efficacy of new treatments for patients. Let’s explore ten common clinical research jobs:
- Clinical Research Coordinator (CRC): CRCs are entry-level roles responsible for assisting with patient recruitment, obtaining informed consent, collecting data, and ensuring protocol adherence. They work closely with patients and research teams to support the smooth implementation of clinical trials.
- Clinical Research Associate (CRA): CRAs also have entry-level positions and are responsible for monitoring clinical trial sites, verifying data, ensuring regulatory compliance, assessing the safety of study subjects, and maintaining study protocols. They play a crucial role in ensuring the integrity and quality of clinical trial data.
- Clinical Trial Manager: As the name suggests, clinical trial managers oversee the operations of clinical trials from start to finish. They manage budgets, coordinate teams of CRAs, and ensure the successful execution of trials. Attention to detail and strong project management skills are essential for this role.
- Clinical Project Manager: Clinical project managers are responsible for managing multiple trials within a program. They collaborate with cross-functional teams, manage resources, align project phases with organizational goals, and ensure the smooth execution of clinical trials. Effective communication and leadership skills are critical in this role.
- Regulatory Affairs Specialist: Regulatory affairs specialists compile, submit, and maintain regulatory documents for approval. They must stay informed about changing regulations and liaise with regulatory agencies to ensure compliance. Attention to detail and an understanding of regulatory requirements are essential in this role.
- Data Manager: Data managers play a vital role in overseeing data collection, cleaning, and database management for clinical trial data. They ensure data quality and compliance with industry standards, contributing to the integrity and accuracy of clinical trial results.
- Clinical Research Scientist: Clinical research scientists design study protocols, analyze data, interpret results, and publish findings in scientific journals. They play a crucial role in advancing medical knowledge and contribute to evidence-based medicine through their research.
- Medical Monitor: Medical monitors focus on patient safety during clinical trials. They review adverse events, assess patient well-being, and recommend study adjustments based on their medical expertise. Their role is crucial in safeguarding the well-being of study participants.
- Clinical Quality Assurance Auditor: Clinical quality assurance auditors conduct inspections, identify non-compliance issues, and recommend corrective actions to ensure adherence to regulations and quality standards. They play an essential role in maintaining the integrity of clinical trial processes.
- Clinical Research Consultant: Clinical research consultants provide expert guidance on study design, regulatory strategies, and data analysis. They work independently or with organizations to solve complex problems and provide valuable insights in the field of clinical research.
These are just a few examples of the diverse career options available in clinical research. Professionals with qualifications or experience in the industry can explore these roles and find rewarding career opportunities in various sectors, including pharma, biotech, medical device companies, and contract research organizations (CROs).
| Key Information | Statistics/Data |
|---|---|
| Clinical Trials Market Size (2020) | $44.3 billion |
| Projected Annual Growth Rate (2021-2028) | 5.7% |
| Participants in English Clinical Research (Apr 2020 – Mar 2021) | 1,390,483 |
As the statistics show, clinical research is a competitive and growing field within the life sciences industry. Professionals in this field can progress to senior roles such as Clinical Director or VP level, depending on their experience and expertise. With the constant demand for innovative treatments and therapies, there is a wealth of opportunities for individuals who are passionate about making a difference in healthcare through clinical research.
Progression in Clinical Research Careers
Progressing in clinical research careers offers exciting opportunities for professionals in the field. By combining experience, education, certifications, and networking, individuals can unlock new levels of career growth and success. Let’s explore some of the potential paths for career progression in clinical research.
1. Clinical Research Coordinator (CRC)
Starting as a Clinical Research Coordinator (CRC) is a common entry point in the field. These professionals oversee the daily operations of clinical trials, ensuring adherence to protocols and regulations. Employers often prefer candidates with a graduate degree, which provides a competitive advantage in the job market. Average salaries for CRCs are around $56,504 per year, with senior positions earning over $89,000.
2. Clinical Research Associate (CRA)
Becoming a Clinical Research Associate (CRA) is a natural career progression for experienced CRCs. CRAs monitor clinical trials, perform site visits, and ensure data integrity. Obtaining a bachelor’s degree, gaining work experience, pursuing a master’s degree or graduate certificate, and obtaining relevant certifications are key steps in advancing as a CRA. Salaries for CRAs vary based on experience levels, with typical ranges as follows:
| Position | Salary Range (per year) |
|---|---|
| CRA I (Clinical Research Associate I) | $50,000 – $70,000 |
| CRA II (Clinical Research Associate II) | $65,000 – $90,000 |
| CRA III / Senior CRA / Lead CRA | $85,000 – $120,000 |
The median salary for a clinical research associate is $83,797 per year, with entry-level positions starting around $48,533 and senior positions reaching approximately $134,940. Salary differences can also depend on the employer, with pharmaceutical companies typically offering higher compensation compared to federal government positions.
3. Specialized Roles and Career Advancement
As professionals gain experience and expertise, they can pursue specialized roles within clinical research. Some potential career paths include:
- Clinical Trial Manager
- Clinical Project Manager
- Director of Clinical Operations
- Regulatory Affairs Manager or Director
- Senior Data Manager or Clinical Data Scientist
- Senior Research Scientist or Director of Clinical Research
- Chief Medical Officer (CMO)
- Senior Auditor or Quality Assurance Manager
These positions often require additional education, such as a master’s degree or advanced certifications, and offer higher salaries and increased responsibilities.
By strategically planning their educational journey, gaining relevant experience, and obtaining certifications like the Clinical Research Coordinator (CCRC), Clinical Research Associate (CCRA), or Certified Clinical Research Professional (CCRP), professionals can enhance their employment opportunities, salary potential, and overall career prospects in the field.
Networking and staying up-to-date with industry trends and advancements also play crucial roles in career progression. Building professional connections, attending conferences, and joining relevant associations like the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SoCRA) can provide valuable opportunities for growth and advancement.
Clinical research careers offer a wealth of possibilities for those passionate about advancing medical research and improving patient outcomes. With dedication, continuous learning, and a strategic approach to career development, professionals can make significant strides in their clinical research career progression.
Furthering Education and Certifications
Continuing education and obtaining certifications play a vital role in advancing your career in clinical research. With the field constantly evolving, staying updated with the latest knowledge and acquiring specialized skills can set you apart from the competition and open doors to new opportunities.
One avenue for furthering your education is pursuing advanced degrees in clinical research or related fields. A Master’s in Clinical Research or an MBA with a concentration in healthcare can deepen your understanding of research methodologies, regulations, and management principles. These advanced degrees can equip you with the necessary skills to take on leadership roles and contribute to the strategic direction of clinical research projects.
Benefits of Obtaining Certifications
In addition to advanced degrees, certifications can enhance your qualifications and increase your marketability in the clinical research industry. Certifications demonstrate your commitment to professional growth and validate your expertise in specific areas.
One prominent certification for Clinical Research Associates (CRAs) is the Certified Clinical Research Professional (CCRP) credential offered by the Society of Clinical Research Associates (SOCRA). The CCRP certification requires a minimum of two years of full-time experience in clinical research and assesses professionals on their knowledge of research ethics, regulatory compliance, study operations, and data management.
For CRAs with at least one year of full-time experience, the International Academy of Clinical Research (IAoCR) offers the Certified Clinical Research Associate (CICRA) credential. This certification focuses on core competencies such as protocol adherence, data collection and analysis, and patient safety.
Impact on Career Advancement and Salaries
Furthering your education and obtaining certifications can significantly impact your career advancement and earning potential in clinical research. By acquiring advanced degrees and certifications, you position yourself for higher-level positions and greater responsibilities, which often come with increased earning potential.
According to statistics, entry-level Clinical Research Coordinators can expect an annual salary ranging from $45,000 to $60,000, while mid-level Coordinators typically earn between $60,000 and $80,000 per year. Senior Coordinators with advanced skills and experience can command salaries exceeding $90,000 annually.
Moreover, geographical location can significantly impact compensation in the clinical research field. Metropolitan areas tend to offer higher salaries compared to rural regions due to factors such as larger research institutions, increased competition, and higher costs of living.
Exploring Certification Programs
When considering certification programs, it is essential to research reputable organizations that offer certifications relevant to your career path. Some well-known organizations in the clinical research industry providing certification programs for CRAs include the Clini Launch Research Institute (CLRI), SOCRA, and IAoCR.
These programs can provide you with the necessary knowledge and credibility to excel in your clinical research career. Additionally, certifications often require continuing education to stay current, enabling you to stay up-to-date with industry trends and best practices.
Investing in your education and obtaining certifications in clinical research can open doors to new opportunities, increase your earning potential, and position you for success in this rapidly growing field. By staying committed to lifelong learning and professional development, you can secure a rewarding and fulfilling career in the diverse and exciting world of clinical research.
| Certification Program | Experience Requirement |
|---|---|
| Certified Clinical Research Professional (CCRP) – SOCRA | At least two years of full-time experience in clinical research |
| Certified Clinical Research Associate (CICRA) – IAoCR | At least one year of full-time experience in clinical research |
The Role of Networking in Career Progression
Networking plays a crucial role in career progression in the clinical research field. By actively building relationships with peers, mentors, and industry professionals, individuals can gain valuable insights and open doors to new opportunities for growth and advancement.
Studies indicate that networking is a powerful tool in the healthcare industry, with a LinkedIn survey estimating that 85% of all jobs are filled through networking. In healthcare, referrals are a key aspect of organizational recruitment strategies, and many hospitals offer robust referral programs with generous incentives designed to encourage employees to refer their contacts.
For professionals in nursing and clinical research, networking events play a crucial role in expanding their professional network and advancing their careers. Whether it’s attending convention center meetings, hospital or company-sponsored events, continuing education seminars, or conferences, these gatherings provide valuable opportunities to connect with peers and industry leaders.
Online networking platforms like LinkedIn also offer nurses and healthcare professionals the chance to connect with others in their field, expand their network, and stay updated on industry trends and opportunities. Being selective about networking sites for nursing or healthcare can enhance the online networking experience, ensuring professionals connect with the right people and engage in meaningful conversations.
Networking not only exposes individuals to different specialties and roles that they may not have previously considered but also helps build alliances for developing processes and policies that support better patient outcomes. By networking and building relationships throughout the healthcare industry, professionals can collaborate with others to create positive change within their field.
When attending networking events, it is essential to set goals beforehand to ensure specific objectives are met. Proper attire at networking events also sets a professional tone and boosts self-assurance, making individuals more approachable to potential connections.
Advancing in Clinical Research Careers through Networking
In the clinical research field, career progression often follows a well-defined path. Starting from entry-level roles like Clinical Trial Assistants (CTA) or Clinical Research Coordinators (CRC), professionals can advance to specialized positions such as Clinical Research Associates (CRA) or Study Start-Up Specialists (SSU) within 1-3 years on average.
Professionals with post-graduate STEM degrees may have the opportunity to skip entry-level positions and directly enter more advanced roles like CRA or SSU. Individuals with 2-5 years of experience in specialized roles can transition to senior positions such as Clinical Trial Managers or Team Leads.
Through specialized industry-bridging programs, graduates can achieve quicker promotions compared to the traditional career path, often in half the time required. After gaining expertise in specific areas like Regulatory Affairs, Clinical Operations, or Data Management for 3-5 years, professionals are typically ready for higher management roles as Subject Matter Experts (SMEs) or Directors.
Senior professionals with seasoned experience may ascend to executive roles like Vice Presidents, Heads of Clinical Operations, or Chief Scientific Officers. Active engagement in industry associations, conferences, and networking events is emphasized to provide opportunities for career advancement and gain valuable industry insights.
Certifications and Leadership Development
In the clinical research field, certifications hold significant value and demonstrate expertise and commitment to professional development. Relevant certifications include Certified Clinical Research Professionals (CCRP), Certified Clinical Research Associates (CCRA), and Clinical Research Coordinator Certification (CRCC).
Additionally, professionals can enhance their career prospects by pursuing advanced degrees such as a master’s degree in clinical research, which can be beneficial for those looking to transition into management or executive-level roles.
Leadership opportunities within an organization also contribute to career growth. Showcasing leadership capabilities and taking on responsibilities can elevate one’s career prospects and open doors to higher-level positions.
Publishing research and pursuing training programs focused on leadership development can also enhance a professional’s value in the clinical research field, setting them apart from their peers and positioning them for future leadership roles.
By recognizing the importance of networking, certifications, and leadership development, clinical research professionals can actively shape their career paths and unlock opportunities for growth and advancement.
Clinical Research Career Progression Paths
In the evolving field of clinical research, professionals have various career progression paths to choose from. These paths are shaped by the individual’s interests, the organization they work for, and their areas of specialization. Over the years, the industry has witnessed a shift towards recognizing the importance of skill development and structured career growth opportunities.
One common career progression path starts with Clinical Research Coordinators (CRCs) who often transition to industry roles such as Clinical Trial Associate (CTA) or Clinical Research Associate (CRA). From there, professionals can advance to senior roles within these positions, gaining more responsibility and leadership opportunities. Other paths include moving into roles such as Clinical Trial Manager, Director of Clinical Operations, Regulatory Affairs Manager, Senior Data Manager, Clinical Research Scientist, Chief Medical Officer (CMO), or Quality Assurance Manager.
Michigan Medicine, for instance, has implemented a career ladder for Clinical Research Coordinators. This ladder consists of market titles like Clinical Research Assistant, Clinical Research Technician, Clinical Research Coordinator Associate, Clinical Research Coordinator Intermediate, Clinical Research Coordinator Senior, Clinical Research Coordinator Lead, and Clinical Research Project Manager. The ladder provides a clear framework for growth and advancement within the organization.
To further enhance their career prospects, professionals in clinical research can pursue certifications such as ACRP CCRC, SoCRA CCRP, or their equivalents. These certifications are often required for positions at the CRC – Associate level or higher. New employees are typically given six months from their start date to acquire certification, and the expense is usually covered by the hiring individual or central unit.
| Competencies |
|---|
| Scientific Concepts |
| Ethical and Participant Safety Concerns |
| Investigational Products Development and Regulations |
| Clinical Study Operations |
| Study and Site Management |
| Data Management and Informatics |
| Leadership and Professionalism |
| Communication and Teamwork |
The outlook for clinical research careers is promising, with an estimated growth rate of 9% per year. This positive trend indicates a growing demand for skilled professionals in the industry. The Joint Task Force Clinical Trial Competency Framework outlines eight distinct domains for acquiring basic competencies in clinical research, ensuring that professionals are well-rounded in their knowledge and skills.
Professionals in clinical research have the advantage of diverse career pathways and the flexibility to switch pathways to reach their ultimate destination. The industry offers various types of employers, including pharmaceutical sponsors, device companies, contract research organizations (CROs), technology and service vendors, regulatory authorities, site networks, and academic institutions. These opportunities provide a wide array of options for professionals to explore and grow in their careers.
The Association of Clinical Research Professionals (ACRP) is a leading professional organization that provides training opportunities for individuals looking to enhance their clinical research skills. These training programs offer valuable resources, networking opportunities, and industry insights to support professionals in their career development.
It’s important to note that job titles in the clinical research industry may vary across organizations, making it challenging to create a standardized pathway map. However, the dynamic landscape of the industry ensures that there are ample opportunities for growth and advancement for those willing to invest in their skills and knowledge.
Clinical Research Consulting Opportunities
For professionals in the clinical research field, there are numerous avenues to explore when it comes to career progression. One such avenue is clinical research consulting, which offers exciting opportunities to provide expert guidance and support to organizations and individuals involved in clinical research.
Clinical research consultants play a pivotal role in assisting with various aspects of the research process. They have extensive knowledge and experience in areas such as study design, regulatory strategies, data analysis, and more. Consulting allows these experts to utilize their skills and expertise to help clients navigate the complexities of clinical research.
As a clinical research consultant, professionals have the flexibility to work independently or collaborate with organizations like Clinical Research Organizations (CROs), Site Management Organizations (SMOs), or even pharmaceutical companies. This allows them to work in different settings and gain exposure to a variety of projects and therapeutic areas.
Clinical research consulting offers several benefits for professionals seeking career growth. By working as a consultant, individuals can broaden their skillset, expand their network, and enhance their industry knowledge. It also provides opportunities to work on innovative research projects and contribute to advancing medical knowledge and improving patient care.
In addition to their clinical research expertise, consultants can also draw on their professional networks to provide value-added services to clients. Networking plays a vital role in consulting, as it allows consultants to connect with industry professionals, build relationships, and attract new opportunities. By collaborating with other experts, consultants can expand their reach and establish themselves as trusted advisors in the field.
To succeed as a clinical research consultant, professionals should consider obtaining advanced degrees, such as a Master’s in Clinical Research or an MBA. These degrees can provide valuable knowledge and skills in business management, project management, and leadership, which are essential for consulting roles.
Certifications can also enhance career growth in clinical research consulting. Credentials like the Clinical Research Professional (CCRP) or Project Management Professional (PMP) demonstrate a consultant’s commitment to professional development and expertise in their respective fields.
Clinical Research Consulting Roles
Clinical research consulting offers a diverse range of roles for professionals to explore. Some potential roles in this field include:
- Clinical Research Consultant
- Regulatory Affairs Consultant
- Data Analysis Consultant
- Study Design Consultant
- Project Management Consultant
| Role | Responsibilities |
|---|---|
| Clinical Research Consultant | Provide expert guidance on all aspects of clinical research, including study design, data analysis, and regulatory compliance. |
| Regulatory Affairs Consultant | Ensure compliance with regulatory requirements and help organizations navigate the complexities of regulatory frameworks. |
| Data Analysis Consultant | Utilize statistical methods and data analysis techniques to interpret research findings and provide meaningful insights. |
| Study Design Consultant | Assist in designing research studies that adhere to ethical standards, optimize data collection, and answer research questions effectively. |
| Project Management Consultant | Provide project management expertise, including oversight of budgets, timelines, and resource allocation for clinical research projects. |
These roles highlight the diverse opportunities available in clinical research consulting. Professionals can choose to specialize in specific areas or offer comprehensive consulting services, depending on their interests and expertise.
In the rapidly evolving field of clinical research, consulting offers an exciting and rewarding career path for professionals looking to make a significant impact. Whether working independently or collaborating with organizations, clinical research consultants play a vital role in ensuring the success of research projects and the advancement of medical knowledge.
Partnership with R&D Partners for Career Success
In the dynamic field of clinical research, partnering with the right organization can make all the difference in achieving career success. One such organization that can provide invaluable support and opportunities is R&D Partners, a leading clinical research staffing agency.
R&D Partners is dedicated to helping individuals excel in their clinical research careers by offering insights, strategies, guidance, and exclusive career opportunities. With their extensive industry knowledge and network, they can assist professionals at every stage of their career, from entry-level positions to senior leadership roles.
What sets R&D Partners apart is their strong focus on building partnerships and collaborations. They understand that in the fast-paced world of clinical research, collaboration is key to driving innovation and advancing medical breakthroughs.
With over 200 active collaborations globally, R&D Partners has established itself as a trusted partner in the industry. They engage in approximately 100 public-private partnerships through R&D across 76 countries, ensuring a global reach for their candidates.
More than 60% of Takeda’s pipeline, a renowned pharmaceutical company, is partnered, highlighting the significance of collaborating for success in the clinical research field. R&D Partners actively supports Takeda in their mission, working closely with their R&D Center for External Innovation (CEI).
The CEI aims to form innovative partnerships, driving external engagement opportunities from early academic research to late-stage clinical collaborations. This presents a wide range of possibilities for professionals looking to advance their careers in clinical research.
Additionally, R&D Partners aligns with Takeda’s focus on innovation by collaborating with Takeda Ventures, Inc., their corporate venture capital group. This strategic partnership allows R&D Partners to invest in innovative life science companies that are in line with Takeda’s R&D focus areas, further expanding career opportunities for their candidates.
Furthermore, R&D Partners recognizes the importance of digital capabilities in the healthcare industry. Through their partnership with Takeda Digital Ventures, they actively invest in companies that leverage digital innovation to improve patient outcomes and care delivery, creating a pathway for professionals to contribute to cutting-edge advancements in the field.
R&D Partners also plays a crucial role in supporting independent science studies through Takeda’s Investigator Initiated Research (IIR) program. This program allows researchers to conduct studies related to Takeda’s compounds and therapeutic areas of interest, further enhancing career opportunities for clinical research professionals.
In collaboration with Kyoto University’s CiRA, T-CiRA, a joint program, focuses on regenerative medicine and drug discovery, shaping the future of healthcare. Through these partnerships, R&D Partners provides professionals with unique opportunities to be at the forefront of groundbreaking research and innovation.
Roles and Opportunities in Clinical Research
Partnering with R&D Partners not only opens doors to exciting collaborations but also provides access to various roles and opportunities in the clinical research industry. Here are some roles that professionals can explore:
| Role | Description |
|---|---|
| Clinical Research Coordinators (CRCs) | Assist with patient recruitment, data collection, and ensuring protocol adherence. |
| Clinical Research Associates (CRAs) | Monitor clinical trial sites, verify data, and assess subject wellbeing. |
| Clinical Trial Managers | Oversee all operations of a clinical trial, managing budgets and timelines. |
| Regulatory Affairs Specialists | Compile and maintain regulatory documents for approval. |
| Data Managers | Oversee data collection, cleaning, and database management for clinical trials. |
| Clinical Research Scientists | Design study protocols, collect and analyze data, and publish findings. |
| Medical Monitors | Review adverse events and oversee patient safety during trials. |
| Clinical Quality Assurance Auditors | Conduct inspections and identify non-compliance issues. |
| Clinical Research Consultants | Provide expert guidance on study design, regulatory strategies, and data analysis. |
These are just a few examples of the diverse roles available in clinical research. R&D Partners can help professionals find the right fit based on their skills, experience, and career aspirations.
By partnering with R&D Partners, individuals can gain access to exclusive job opportunities, expert guidance, and a network of industry professionals. R&D Partners’ collaborative approach to career development can significantly contribute to the success and growth of clinical research professionals.
Clinical Research Career Paths Infographic
Proclinical Infographics offers a comprehensive visual representation of the diverse career paths in clinical research. This infographic highlights the various options available for professionals looking to enter or advance within the field.
With the growing demand for skilled clinical research professionals, it’s essential to understand the different roles and opportunities available. The infographic outlines the key positions and responsibilities within the clinical research industry, providing valuable insights into career progression.
Let’s take a closer look at some of the roles showcased in the infographic:
Clinical Trial Manager:
The Clinical Trial Manager plays a pivotal role in overseeing the budget of a clinical trial. They ensure that resources are allocated efficiently and effectively, ultimately contributing to the success of the trial.
Senior Clinical Research Associate I-III:
Senior Clinical Research Associates (CRAs) are responsible for monitoring study sites and activities. They ensure compliance with protocol, regulations, and ethical standards while maintaining data integrity and participant safety.
Clinical Research Associate I-III:
Clinical Research Associates evaluate and interpret collected study data, providing critical insights that contribute to the overall success of the research. They ensure that data is accurate, complete, and compliant with regulatory requirements.
Clinical Trial Assistant:
The Clinical Trial Assistant plays a crucial role in managing and developing clinical trial teams. They support the performance and development of the team, ensuring smooth operations and efficient execution of trials.
In addition to the roles mentioned above, the infographic highlights positions such as Director of Clinical Team Management, Director of Clinical Trial Management, Director of Clinical Trial Project Management, VP/Head of Clinical Operations, and Clinical Project Manager. These roles involve strategic planning, budget management, and overseeing the overall operations and success of clinical trials and research projects.
It’s important to note that the path to a successful clinical research career involves acquiring the necessary qualifications and skills. Companies may offer structured training programs or external courses to equip individuals with the knowledge and expertise required in the field.
Additionally, professionals in the clinical research industry have various opportunities for further education and certifications. Diplomas or graduate certificates in clinical research can enhance job prospects and open doors to advanced roles.
Stay informed about global trends in clinical research by actively engaging with industry publications, attending webinars, and participating in ongoing education programs. Building a strong professional network is also crucial for career progression and staying up-to-date with the latest developments in the field.
As the demand for clinical research professionals continues to rise, it’s important to highlight your relevant skills and experience when applying for job opportunities. Emphasizing your attention to detail, effective communication, ethical conduct, data management, and regulatory knowledge can make you stand out in the competitive field of clinical research.
With a solid foundation in life sciences or a relevant discipline, a bachelor’s degree is typically the minimum requirement for entry-level positions in the clinical research field. However, pursuing a Master’s or Ph.D. degree can unlock advanced roles and provide opportunities for career growth.
The infographic serves as a valuable resource for understanding the multifaceted career paths available in clinical research. Whether you’re starting your journey or seeking advancement, it’s essential to explore the various options and tailor your career path to your strengths and aspirations.
Becoming a Clinical Director or VP
With the right experience and qualifications, clinical professionals have the potential to progress to higher-level positions such as clinical director or even Vice President (VP). These roles offer significant opportunities for career growth and the chance to make a lasting impact on the field of clinical research.
To become a clinical director or VP, professionals often need a combination of relevant education, experience, and demonstrated leadership abilities. Common educational backgrounds for clinical directors include bachelor’s degrees (43.3%), master’s degrees (32.7%), and associate degrees (10.8%). Gaining advanced degrees in fields such as healthcare administration, clinical research, or related fields can enhance the skills and knowledge required for these roles.
Key competencies for clinical directors include a deep understanding of patients (21.01%), clinical trials (12.36%), oversight (6.82%), and regulatory guidelines such as FDA (6.08%) and GCP (6.06%). Strong communication, organization, multitasking, and critical thinking skills are also necessary to effectively lead and manage clinical research teams.
Transitioning to the role of clinical director or VP is often a result of progression within the field and gaining experience in roles such as staff nurse, therapist team lead, or registered nurse for about 4-6 years. This experience allows professionals to develop the necessary expertise and leadership skills required for higher-level positions.
As a clinical director or VP, professionals play a crucial role in overseeing and leading clinical research teams. They are responsible for making strategic decisions, ensuring compliance with regulations, managing budgets, and contributing to the overall success of research studies. Additionally, they may be involved in developing and implementing research strategies, collaborating with cross-functional teams, and driving innovation in the field of clinical research.
The average yearly salary for a clinical director is $96,497 or $46.39 hourly, making these positions highly rewarding both professionally and financially. Job satisfaction ratings for clinical directors vary, with many reporting positive experiences related to staff growth and department development.
Overall, becoming a clinical director or VP offers clinical professionals the opportunity to advance their careers, have a significant impact on the healthcare industry, and contribute to the development of new treatments and therapies. With the right combination of education, experience, and skills, individuals can embark on a fulfilling and rewarding journey in clinical research leadership.
Image related to the field of Clinical Research
Clinical trial management career path.
The clinical trial management career path offers a rewarding and dynamic opportunity for individuals interested in the field of clinical research. Clinical trial managers play a crucial role in managing, coordinating, and overseeing all aspects of clinical trials.
With a bachelor’s degree being the most common qualification held by clinical trial managers, it is evident that education plays a significant role in this career path. Approximately 60.13% of clinical trial managers hold a bachelor’s degree, while 21.8% have a master’s degree and some hold a doctorate.
As a clinical trial manager, responsibilities encompass various areas, including patient-related tasks, clinical trial management, clinical operations, clinical research, and oversight. Patient-related tasks account for 7.63% of the clinical trial manager’s responsibilities, while clinical trial management itself comprises 6.83% of their duties. Clinical operations and clinical research account for 6.27% and 6.23%, respectively, while oversight tasks represent 5.99% of the job responsibilities.
One of the key factors that make the clinical trial management career path enticing is the average yearly salary. Clinical trial managers earn an average salary of $93,644, making it an attractive field for those seeking financial stability. Additionally, the job is highly regarded within the industry, with a general rating of 5 stars.
However, it is essential to consider the challenges associated with the role. A user review of a clinical trial manager noted high stress and a lack of people interaction as cons of the job. While the career path offers numerous benefits, it is crucial to be aware of the potential challenges and ensure personal suitability.
To further advance in the clinical trial management career path, professionals can pursue certifications provided by reputable organizations. The Association of Clinical Research Professionals (ACRP) offers various certifications, including ACRP Certified Professional (ACRP-CP), Clinical Research Coordinator (CCRC), Clinical Research Associate (CCRA), Principal Investigator (CPI), ACRP Medical Device Professional Subspecialty (ACRP-MDP), and ACRP Project Manager Subspecialty. The Society of Clinical Research Associates (SOCRA) also provides the Certified Clinical Research Professional credential.
In terms of career opportunities, clinical trial coordinators can find employment in a range of settings, such as health care organizations, pharmaceutical companies, research hospitals, and government and private companies. The demand for skilled professionals in this field is significant, and job prospects are promising.
While a bachelor’s degree is typically the minimum education requirement to work as a clinical trial manager, having a master’s degree or a graduate certificate can set candidates apart. Work experience in health care or clinical research is also crucial for most positions.
Individuals interested in pursuing a clinical trial management career path should consider obtaining relevant education in subjects such as clinical research administration, health sciences, public health, microbiology, clinical research management, and medical technology. These areas of study will provide a solid foundation for success in this field.
Overall, the clinical trial management career path offers a fulfilling and challenging journey for those passionate about clinical research. It provides opportunities for personal and professional growth, lucrative salaries, and the chance to contribute to advancements in medical science.
Clinical Trial Management Career Path Statistics
| Statistic | Percentage/Value |
|---|---|
| Percentage of clinical trial managers with a bachelor’s degree | 60.13% |
| Percentage of clinical trial managers with a master’s degree | 21.8% |
| Percentage of clinical trial managers with a doctorate | Not specified |
| Patient-related tasks as a percentage of clinical trial manager responsibilities | 7.63% |
| Clinical Trial Management as a percentage of clinical trial manager duties | 6.83% |
| Clinical Operations as a percentage of clinical trial manager duties | 6.27% |
| Clinical Research as a percentage of clinical trial manager responsibilities | 6.23% |
| Oversight tasks as a percentage of clinical trial manager job duties | 5.99% |
| Average yearly salary for a Clinical Trial Manager | $93,644 |
| General rating given to the Clinical Trial Manager job | 5 Stars |
| Cons of the Clinical Trial Manager role mentioned in a user review | High stress and lack of people interaction |
Clinical Team Management Career Path
Within the field of clinical research, the clinical team management career path offers a rewarding and dynamic opportunity for individuals to lead and support team members in the pursuit of innovative medical advancements. This career path focuses on managing the day-to-day operations of clinical trials and ensuring the smooth execution of research protocols.
Clinical team managers are responsible for acting as direct line managers to associates, providing guidance and mentorship, and ensuring effective training and development opportunities. They review business processes and implement improvements to enhance operational efficiency. Additionally, they play a crucial role in planning and organizing professional development activities to foster growth within the team.
Key Responsibilities:
- Leading and managing team members involved in clinical research projects
- Ensuring effective training and development of team members
- Reviewing and optimizing business processes
- Planning and organizing professional development activities
A career in clinical team management allows individuals to cultivate and showcase their leadership skills. The ability to effectively manage teams, navigate complex protocols, and drive collaboration among various stakeholders is essential in this role.
By pursuing a clinical team management career path, individuals have the opportunity to make a significant impact on the advancement of healthcare. They contribute to the successful execution of clinical trials and play a vital role in bringing life-changing treatments to patients.
With the rapid growth of the clinical research market, there is an increasing demand for skilled professionals in clinical team management. Organizations value individuals who possess a solid educational foundation, hands-on experience, and relevant certifications. Most positions in clinical development require at least a bachelor’s degree in fields such as life sciences, pharmacy, or nursing. Certain roles, such as clinical project managers, may necessitate a master’s degree or higher education.
Professional certifications from reputable organizations, such as the Society of Clinical Research Associates (SOCRA) and the Association of Clinical Research Professionals (ACRP), can enhance credibility in the job market and demonstrate a commitment to professional development.
While specific licenses may be required for certain roles, gaining hands-on experience through internships, research assistant positions, or entry-level roles is crucial when pursuing a career in clinical team management. This hands-on experience provides valuable insights into the intricacies of clinical research and helps individuals develop the skills necessary for effective team management.
Key Roles in Clinical Team Management:
- Clinical Research Associate
- Clinical Data Manager
- Clinical Trials Manager
As the clinical research field continues to expand, the job market for clinical team management professionals remains strong. The United States Bureau of Labor Statistics predicts a 14% annual increase in Clinical Research Associate (CRA) positions from 2014 to 2024, indicating significant job opportunities.
| Job Title | Starting Salary | Salary after 1-3 Years of Experience |
|---|---|---|
| Clinical Study Coordinator (CRC) | $40,000 – $60,000 per year | $50,000 – $75,000 per year |
| Clinical Research Associate (CRA) | $60,000 – $80,000 per year | $75,000 – $100,000 per year |
| Clinical Trial Assistant (CTA) | $35,000 – $50,000 per year | $40,000 – $60,000 per year |
| Study Start-Up Specialist | $50,000 – $70,000 per year | $60,000 – $90,000 per year |
| Trial Master File (TMF) Specialist | $50,000 – $70,000 per year | $60,000 – $90,000 per year |
It’s important to note that career progression and salary growth in clinical team management depend on factors such as experience, education level, certifications, and performance. Individuals who continuously pursue professional development and expand their skill set have greater opportunities for advancement in this field.
Boston Scientific, a renowned company in the healthcare industry, exhibits low turnover rates among CRAs, indicating job stability and satisfaction within the organization. This highlights the potential for long-term career growth and fulfilling opportunities in clinical team management.
Aspiring professionals in clinical team management can further their education by pursuing master’s degree programs in clinical research, offered by esteemed institutions like Eastern Michigan University. These programs provide additional training opportunities and specialized knowledge to individuals aiming to excel in their careers.
To enhance their qualifications and marketability, CRAs often pursue clinical research certifications such as SOCRA’s Certified Clinical Research Professional (CCRP®) or the Association of Clinical Research Professionals-Certified Professional (ACRP-CP). These certifications validate their expertise, demonstrate a commitment to best practices, and showcase their dedication to advancing the field of clinical research.
Travel and Demands:
It’s important to consider that individuals in clinical team management roles, such as CRAs, spend a significant amount of time traveling to various research sites, covering different regions and states. This aspect of the job may impact their personal lives and relationships. However, for those who enjoy a dynamic work environment and embrace the challenges of traveling, this career path offers unique experiences and the opportunity to collaborate with diverse teams and communities.
Companies like Boston Scientific recognize the significance of proper onboarding and training for new CRAs. Their comprehensive onboarding process involves four to six months of training, ensuring that new hires receive thorough preparation and are equipped with the necessary skills to excel in their roles.
The clinical team management career path offers professionals the chance to contribute to groundbreaking medical advancements, drive team success, and make a tangible difference in patients’ lives. With immense job opportunities, solid salary prospects, and avenues for professional growth, individuals passionate about clinical research and team management can embark on a rewarding and fulfilling career journey.
Clinical Project Management Career Path
The clinical project management career path offers a rewarding and dynamic role in the field of clinical research. With the increasing demand for qualified professionals in clinical trial management and clinical project management, this career path presents numerous opportunities for growth and advancement.
Clinical project managers play a crucial role in planning and directing clinical trials. They are responsible for evaluating clinical data, maintaining study records, ensuring activities are delivered on time and budget, and developing trial plans. Their expertise and leadership are essential in overseeing the beginning and end of clinical research studies.
To pursue a career in clinical project management, a Bachelor’s degree in medicine, science, or a relevant field is usually required. Some employers may prefer candidates with a master’s level degree, as it can facilitate easier advancement in the field. Additionally, having five or more years of experience in a relevant field is typically necessary to secure a position as a clinical project manager.
The primary difference between a clinical trial manager and a clinical project manager lies in the scope of their responsibilities. Clinical trial managers focus primarily on managing specific elements of clinical trials, while clinical project managers oversee entire drug or medical device trials across all functions. As a clinical project manager, you will have a diverse range of responsibilities, including drafting and checking paperwork related to clinical projects, reporting and summarizing data for regulatory purposes, maintaining thorough trial records, and determining participant eligibility.
Alongside technical skills, certain soft skills are essential for success in the clinical project management career path. Effective communication skills, problem-solving abilities, interpersonal skills, adaptability, and time management are highly valued in this role. Staying updated with industry trends, possessing good presentation skills, cultural sensitivity, and a continuous enthusiasm for learning are also competencies that can set a clinical project manager apart from others in the field.
Obtaining relevant certifications can further enhance your career prospects and demonstrate your commitment to professional growth. Some of the top certifications for clinical project managers include Project Manager Professional (PMP), Certified Clinical Research Professional (CCRP), Certified Clinical Project Manager (CCPM), Certification in Clinical Research (CCR), and Regulatory Affairs Certification (RAC).
The timeline to become a clinical project manager typically ranges from 2 to 5 years, depending on individual qualifications and experience. Once the necessary experience is gained, building a strong portfolio and resume is crucial for applying to clinical project management positions.
Clinical Project Management Career Path: Key Highlights
| Median Annual Salary (Feb 2021) | Salary Range | Required Experience |
|---|---|---|
| $92,000 | $61,000 – $133,000 | 5+ years in a relevant field |
In terms of salary, clinical project managers had a median annual salary of $92,000 in February 2021, with a salary range from $61,000 for the lowest earners to over $133,000 for the highest earners. Experienced clinical project managers earned about $125,500, showcasing the impact of experience on salary growth.
Moreover, the job outlook for clinical project managers is promising. The U.S. Bureau of Labor Statistics (BLS) projects a 32% job growth for medical and health services managers, including clinical project managers, between 2019 and 2029, surpassing the average job growth for all occupations during the same period.
Overall, the clinical project management career path offers a challenging and impactful role in the field of clinical research. With increasing demand, competitive salaries, and opportunities for professional growth, it is an excellent choice for individuals passionate about managing clinical trials and making a difference in the healthcare industry.
In conclusion, a career in clinical research offers promising prospects for individuals interested in making a meaningful impact in the field of medicine. With the increasing demand for skilled clinical researchers in Alberta, Canada, and the conducive environment created by numerous pharmaceutical companies, research institutions, and healthcare facilities in Calgary, there are ample opportunities for career growth and specialization.
Professionals in clinical research have the chance to work on international projects as clinical trials become more globalized. With diverse career paths available, including roles in clinical operations, regulatory affairs, data management, and medical writing, individuals can pursue their areas of interest and experience professional growth.
To excel in the field, acquiring certifications such as the Certified Clinical Research Professional (CCRP) and joining professional organizations like the Association of Clinical Research Professionals (ACRP) can enhance networking opportunities and industry connections. Furthering education, such as pursuing a Master’s degree in Clinical Research or a related field, can also open doors to higher-level positions.
Overall, a successful clinical research career requires tenacity, industry experience, and preferably a PhD or a Life Sciences degree. By gaining experience across different types of organizations and specializing in specific fields such as oncology, cardiology, or neurology, individuals can progress and reach roles like Clinical Research Lead, Clinical Project Manager, Clinical Specialist, and even Director.
What career opportunities are available in clinical research?
How can i progress in my clinical research career, is furthering education beneficial for a career in clinical research, how important is networking for career progression in clinical research, what career paths can i take in clinical research, what do clinical research consultants do, how can r&d partners help in my clinical research career, where can i find an infographic on clinical research career paths, what are the potential career progression options beyond clinical research roles, what responsibilities are involved in a clinical trial management career path, what does a clinical team management career path entail, what are the responsibilities of a clinical project management career path, what are the career opportunities in the field of clinical research, leave a comment cancel reply.
Save my name, email, and website in this browser for the next time I comment.
Welcome to this career-focused blog, a dedicated space for exploring the dynamic and evolving world of career industry. This platform delves into the intricacies of professional development, industry trends, and practical strategies for career advancement. This is a place for those who are passionate about their professional growth and eager to stay ahead in a rapidly changing landscape.
Recent Posts

Warehouse Manager Career Path: A Complete Guide

Chief of Staff Career Path: A Complete Guide

Technician Career Path: A Complete Guide

Clinical Research Associate Career Path: A Complete Guide

Strategic Finance Career Path: A Complete Guide
CFO Career Path Chart: A Complete Guide

Chef Career Path: A Complete Guide

Security Guard Career Path: A Complete Guide

Staff Accountant Career Path: A Complete Guide

SDR Career Path: A Complete Guide

7 Steps To Becoming A Clinical Research Coordinator

7 Steps to Launching Your Career as a Clinical Research Coordinator
The prospect of a career in clinical research can be exciting , especially for those with a passion for science, medicine, and helping others. A Clinical Research Coordinator (CRC) plays a vital role in this field, ensuring research is conducted ethically and efficiently. If this sounds like the path for you, here are 7 essential steps to becoming a successful CRC:
Earn a Relevant Degree:
A bachelor's degree in a science-related field like biology, chemistry, or healthcare administration is typically required ( National Institutes of Health .gov ). Some employers may prefer a master's degree for more specialized roles ( National Institutes of Health.gov ). Consider exploring the Clinical Research Coordinator course for targeted training in this role.
Gain Hands-on Experience:
Internships or entry-level positions in clinical research settings offer invaluable experience ( Association of Clinical Research Professionals (ACRP) ). This practical exposure strengthens your resume and provides real-world knowledge for future CRC roles. Gain further insights through the Clinical Trials Assistant Training course .
Consider Certification:
While not always mandatory, CRC certification enhances your credentials and marketability. Programs like those offered by the ACRP validate your expertise and set you apart from other candidates. Expand your certification options with the CRA course and the ICH-GCP course .
Develop Core Skills:
Crucial skills for CRCs include: attention to detail, organization, critical thinking, and effective communication. A strong understanding of research regulations and ethics is also crucial. Enhance these skills through the Advanced Clinical Research Project Manager Certification .
Build Your Network:
Attend industry events, conferences, and seminars to connect with professionals. Networking opens doors to opportunities, mentorship, and valuable industry insights. Consider further specialization with the Advanced Principal Investigator Physician Certification .
Apply for CRC Positions:
With your qualifications, certifications, and experience in place, actively seek CRC positions. Tailor your resume to highlight relevant skills and experiences, and craft a compelling cover letter showcasing your passion for research. Research the organization and demonstrate your knowledge during interviews.
Embrace Continuous Learning:
The field of clinical research is constantly evolving. Stay informed about industry trends, participate in continuing education, and pursue professional development opportunities to stay ahead in your CRC career. The Pharmacovigilance Certification and the Medical Monitor Certification can be instrumental in your continuous learning journey.
References:
National Institutes of Health (.gov): https://toolkit.ncats.nih.gov/glossary/clinical-research-coordinator/
Association of Clinical Research Professionals (ACRP): https://acrpnet.org/
Society for Clinical Research Associates (SCRA): https://www.scra.org/
How to Be a Clinical Research Coordinator
A description of clinical research coordinator jobs and what they entail.
You are using an outdated browser. Please upgrade your browser to improve your experience.
Health & Nursing
Courses and certificates.
- Bachelor's Degrees
- View all Business Bachelor's Degrees
- Business Management – B.S. Business Administration
- Healthcare Administration – B.S.
- Human Resource Management – B.S. Business Administration
- Information Technology Management – B.S. Business Administration
- Marketing – B.S. Business Administration
- Accounting – B.S. Business Administration
- Finance – B.S.
- Supply Chain and Operations Management – B.S.
- Communications – B.S.
- User Experience Design – B.S.
- Accelerated Information Technology Bachelor's and Master's Degree (from the School of Technology)
- Health Information Management – B.S. (from the Leavitt School of Health)
Master's Degrees
- View all Business Master's Degrees
- Master of Business Administration (MBA)
- MBA Information Technology Management
- MBA Healthcare Management
- Management and Leadership – M.S.
- Accounting – M.S.
- Marketing – M.S.
- Human Resource Management – M.S.
- Master of Healthcare Administration (from the Leavitt School of Health)
- Data Analytics – M.S. (from the School of Technology)
- Information Technology Management – M.S. (from the School of Technology)
- Education Technology and Instructional Design – M.Ed. (from the School of Education)
Certificates
- Supply Chain
- Accounting Fundamentals
- Digital Marketing and E-Commerce
- View all Business Degrees
Bachelor's Preparing For Licensure
- View all Education Bachelor's Degrees
- Elementary Education – B.A.
- Special Education and Elementary Education (Dual Licensure) – B.A.
- Special Education (Mild-to-Moderate) – B.A.
- Mathematics Education (Middle Grades) – B.S.
- Mathematics Education (Secondary)– B.S.
- Science Education (Middle Grades) – B.S.
- Science Education (Secondary Chemistry) – B.S.
- Science Education (Secondary Physics) – B.S.
- Science Education (Secondary Biological Sciences) – B.S.
- Science Education (Secondary Earth Science)– B.S.
- View all Education Degrees
Bachelor of Arts in Education Degrees
- Educational Studies – B.A.

Master of Science in Education Degrees
- View all Education Master's Degrees
- Curriculum and Instruction – M.S.
- Educational Leadership – M.S.
- Education Technology and Instructional Design – M.Ed.
Master's Preparing for Licensure
- Teaching, Elementary Education – M.A.
- Teaching, English Education (Secondary) – M.A.
- Teaching, Mathematics Education (Middle Grades) – M.A.
- Teaching, Mathematics Education (Secondary) – M.A.
- Teaching, Science Education (Secondary) – M.A.
- Teaching, Special Education (K-12) – M.A.
Licensure Information
- State Teaching Licensure Information
Master's Degrees for Teachers
- Mathematics Education (K-6) – M.A.
- Mathematics Education (Middle Grade) – M.A.
- Mathematics Education (Secondary) – M.A.
- English Language Learning (PreK-12) – M.A.
- Endorsement Preparation Program, English Language Learning (PreK-12)
- Science Education (Middle Grades) – M.A.
- Science Education (Secondary Chemistry) – M.A.
- Science Education (Secondary Physics) – M.A.
- Science Education (Secondary Biological Sciences) – M.A.
- Science Education (Secondary Earth Science)– M.A.
- View all Technology Bachelor's Degrees
- Cloud Computing – B.S.
- Computer Science – B.S.
- Cybersecurity and Information Assurance – B.S.
- Data Analytics – B.S.
- Information Technology – B.S.
- Network Engineering and Security – B.S.
- Software Engineering – B.S.
- Accelerated Information Technology Bachelor's and Master's Degree
- Information Technology Management – B.S. Business Administration (from the School of Business)
- User Experience Design – B.S. (from the School of Business)
- View all Technology Master's Degrees
- Cybersecurity and Information Assurance – M.S.
- Data Analytics – M.S.
- Information Technology Management – M.S.
- MBA Information Technology Management (from the School of Business)
- Full Stack Engineering
- Web Application Deployment and Support
- Front End Web Development
- Back End Web Development
3rd Party Certifications
- IT Certifications Included in WGU Degrees
- View all Technology Degrees
- View all Health & Nursing Bachelor's Degrees
- Nursing (RN-to-BSN online) – B.S.
- Nursing (Prelicensure) – B.S. (Available in select states)
- Health Information Management – B.S.
Health and Human Services – B.S.
- Psychology – B.S.
Health Science – B.S.
- Public Health – B.S.
- Healthcare Administration – B.S. (from the School of Business)
- View all Nursing Post-Master's Certificates
- Nursing Education—Post-Master's Certificate
- Nursing Leadership and Management—Post-Master's Certificate
- Family Nurse Practitioner—Post-Master's Certificate
- Psychiatric Mental Health Nurse Practitioner —Post-Master's Certificate
- View all Health & Nursing Degrees
- View all Nursing & Health Master's Degrees
- Nursing – Education (BSN-to-MSN Program) – M.S.
- Nursing – Leadership and Management (BSN-to-MSN Program) – M.S.
- Nursing – Nursing Informatics (BSN-to-MSN Program) – M.S.
- Nursing – Family Nurse Practitioner (BSN-to-MSN Program) – M.S. (Available in select states)
- Nursing – Psychiatric Mental Health Nurse Practitioner (BSN-to-MSN Program) – M.S. (Available in select states)
- Nursing – Education (RN-to-MSN Program) – M.S.
- Nursing – Leadership and Management (RN-to-MSN Program) – M.S.
- Nursing – Nursing Informatics (RN-to-MSN Program) – M.S.
Master of Healthcare Administration
Master of Public Health
- MBA Healthcare Management (from the School of Business)
- Business Leadership (with the School of Business)
- Supply Chain (with the School of Business)
- Accounting Fundamentals (with the School of Business)
- Digital Marketing and E-Commerce (with the School of Business)
- Back End Web Development (with the School of Technology)
- Front End Web Development (with the School of Technology)
- Web Application Deployment and Support (with the School of Technology)
- Full Stack Engineering (with the School of Technology)
- Single Courses
- Course Bundles
Apply for Admission
Admission requirements.
- New Students
- WGU Returning Graduates
- WGU Readmission
- Enrollment Checklist
- Accessibility
- Accommodation Request
- School of Education Admission Requirements
- School of Business Admission Requirements
- School of Technology Admission Requirements
- Leavitt School of Health Admission Requirements
Additional Requirements
- Computer Requirements
- No Standardized Testing
- Clinical and Student Teaching Information
Transferring
- FAQs about Transferring
- Transfer to WGU
- Transferrable Certifications
- Request WGU Transcripts
- International Transfer Credit
- Tuition and Fees
- Financial Aid
- Scholarships
Other Ways to Pay for School
- Tuition—School of Business
- Tuition—School of Education
- Tuition—School of Technology
- Tuition—Leavitt School of Health
- Your Financial Obligations
- Tuition Comparison
- Applying for Financial Aid
- State Grants
- Consumer Information Guide
- Responsible Borrowing Initiative
- Higher Education Relief Fund
FAFSA Support
- Net Price Calculator
- FAFSA Simplification
- See All Scholarships
- Military Scholarships
- State Scholarships
- Scholarship FAQs
Payment Options
- Payment Plans
- Corporate Reimbursement
- Current Student Hardship Assistance
- Military Tuition Assistance
WGU Experience
- How You'll Learn
- Scheduling/Assessments
- Accreditation
- Student Support/Faculty
- Military Students
- Part-Time Options
- Virtual Military Education Resource Center
- Student Outcomes
- Return on Investment
- Students and Gradutes
- Career Growth
- Student Resources
- Communities
- Testimonials
- Career Guides
- Skills Guides
- Online Degrees
- All Degrees
- Explore Your Options
Admissions & Transfers
- Admissions Overview
Tuition & Financial Aid
Student Success
- Prospective Students
- Current Students
- Military and Veterans
- Commencement
- Careers at WGU
- Advancement & Giving
- Partnering with WGU
HEALTHCARE CAREER GUIDES
Clinical Research Coordinator Career
What is a clinical research coordinator.
Clinical research coordinators (CRCs) manage everything from participant recruitment to data collection. They’re responsible for directing the day-to-day activities involved in a diverse range of scientific inquiries, including drug trials, epidemiological investigations, genetic testing, and observational studies. CRCs help maintain a study's overall quality and integrity, ensuring that all systems and procedures adhere to informed consent laws, ethical standards, and federal regulations established by the Food and Drug Administration (FDA). Another central aspect of their work involves facilitating communication between the research team and the Institutional Review Board (IRB), an administrative body that safeguards participants’ rights. Before an organization can initiate a study, research coordinators must submit the study to the IRB for approval and make any requested modifications. A clinical research coordinator’s job is highly collaborative. Working closely with principal investigators, study sponsors, and regulatory agencies, these professionals promote goal alignment and foster a spirit of teamwork throughout the research process.
RESPONSIBILITIES
What Does a Clinical Research Coordinator Do?
Clinical research professionals’ work responsibilities can vary. A typical day often involves the following tasks:
- Recruiting patients to participate in clinical trials and research studies.
- Explaining the risks and potential benefits to participants so they can provide informed consent.
- Answering patients’ questions and addressing any of their concerns.
- Preparing and submitting reports detailing research practices to the IRB and FDA.
- Scheduling appointments and medical procedures.
- Ensuring that clinical studies comply with all relevant laws and regulations.
- Enabling smooth communication between the study subjects and the clinical staff.
- Conducting baseline assessments and patient interviews.
- Collecting and organizing data, including patient medical histories, study procedures, and test results.
- Maintaining updated documentation for regulatory authorities.
- Drafting reports about adverse events and protocol deviations.
- Managing the inventory of laboratory supplies and equipment.
- Assisting with grant applications, expense tracking, and participant reimbursement.

Where Does a Clinical Research Coordinator Work?
Clinical research coordinators play a crucial role in the healthcare industry. These professionals work in a variety of settings, including:
- Academic medical centers
- Universities
- Pharmaceutical companies
- Biotech companies
- Private research clinics
- Government agencies
- Nonprofit organizations
EDUCATION & BEST DEGREES
How do i become a clinical research coordinator .
The educational requirements for a clinical research coordinator position can differ based on the organization and job responsibilities. Employers typically seek candidates with at least a bachelor’s degree in a science or medical field. Many clinical research coordinators have educational backgrounds in public health, biology, health science , health and human services , biomedical technology, or healthcare administration . A graduate degree such as a master’s in public health may be required for senior positions or specialized roles. Individuals seeking to enhance their expertise and career prospects can also pursue professional certifications such as the Certified Clinical Research Professional (CCRP) and Certified Clinical Research Coordinator (CCRC) certifications.

Best Degrees for a Clinical Research Coordinator
An online health degree program for students who are committed to making a...
An online health degree program for students who are committed to making a difference for patients in a variety of ways.
- Time: 63% of students finish this program in 24 months
- Tuition: $4,210 per 6-month term
- Courses: 35 total courses in this program
Skills for your résumé that you will learn in this program:
- Epidemiology
- Community and Public Health
- Cultural Awareness
- Pathophysiology
- Healthcare Values and Ethics
- Substance Abuse Support
This degree allows you to work inside the healthcare industry, while also directly working with patients who need help.
An online health science program designed for students who want real-world...
An online health science program designed for students who want real-world skills for valuable health careers.
- Time: 63% of students finish similar programs in 24 months.
- Courses: 28 total courses in this program
- Disease prevention
- Behavioral health
- Substance abuse support
- Health research
- Medical technology
This degree prepares you with relevant industry skills and experience that will help you move forward in your healthcare career.
A master's focused on managing comprehensive, value-based care, directly...
A master's focused on managing comprehensive, value-based care, directly in line with innovations in health and healthcare.
- Time: 60% of grads finish within 21 months.
- Tuition: $4,995 per 6-month term.
- Courses: 12 total courses in this program.
- Collaborative Leadership
- Healthcare Models and Systems
- Healthcare Financial Management
- Enterprise Risk Management
- Healthcare Information Technology
Your rich experience in a health-related field can mean more when you bring a master's level of understanding to the problems that organizations need to solve.
Compare degrees
This program is not the only degree WGU offers designed to create leaders in the field of healthcare. Compare our health leadership degrees.
This online Master of Public Health degree program is a perfect fit for...
This online Master of Public Health degree program is a perfect fit for students who want to make a difference in their community.
- Time: 60% of students finish similar programs in 21 months.
- Tuition: $4,995 per 6-month term
- Courses: 12 total courses in this program
Skills for your résumé you will learn in this program:
- Biostatistics and analysis
- Environmental health
- Global health
- Public health policy and advocacy
- Health education and promotion
This degree prepares you with relevant industry skills and experience that will help you move forward in your career.
Public Health - B.S.
This online bachelor's degree in public health will prepare you to impact...
This online bachelor's degree in public health will prepare you to impact your community and make a difference.
- Courses: 33 total courses in this program
- Cognitive psychology
- Public health approaches
- Biopsychosocial health models

How Much Does a Clinical Research Coordinator Make?
According to Salary.com, the average annual salary for clinical research coordinators is $69,974 . Professionals in this field typically earn between $60,108 and $80,825 a year. However, the top 10% of earners can make more than $90,705. Salaries can vary depending on several factors, including geographic location, industry, work experience, certifications, and education. Clinical research coordinators with significant on-the-job experience can expect to earn higher wages than those just starting their careers.

What Is the Job Outlook for a Clinical Research Coordinator?
Advancements in technology and increased funding for scientific research have led to a growing demand for clinical research coordinators who can manage medical studies. From 2022 to 2032, the job growth for natural sciences managers, including research coordinators, is projected to increase by 5% . This is nearly twice the average growth rate for all occupations, meaning the job outlook for clinical research coordinators is favorable. According to the Bureau of Labor Statistics (BLS), there will be about 6,500 clinical research coordinator openings each year during this period.
What Skills Does a Clinical Research Coordinator Need?
Because the role involves a diverse set of responsibilities, clinical research coordinators need a combination of communication abilities, technical expertise, and managerial skills. Critical aptitudes for this job include:
- Technological proficiency. Research institutions use digital management systems, electronic investigator site files, electronic health record systems, and other technologies to automate tasks and organize information.
- Interpersonal communication. Clinical research coordinators utilize excellent communication skills to explain complex study protocols to subjects and to collaborate with interdisciplinary teams.
- Cultural sensitivity. Because they work with patients from diverse backgrounds, clinical research coordinators must understand cultural nuances and be respectful of differing beliefs.
- Medical knowledge. A basic understanding of medical terminology and healthcare practices helps facilitate smooth communication between research coordinators, investigators, and patients.
- Data management. Because they manage vital details about participants and procedures, clinical research coordinators should be adept at collecting and organizing data.
- Regulatory knowledge. To ensure compliance, clinical research coordinators need a comprehensive understanding of the laws, regulations, and standards involved in clinical research.
- Writing. Clinical research coordinators compose reports about research progress, adverse events, study outcomes, and compliance issues.
- Organization. Keeping orderly records of appointment schedules, research procedures, regulatory documentation, assessment data, and other information is essential to a research coordinator’s job.
- Time management. Clinical research coordinators often direct multiple studies simultaneously, so they must prioritize tasks and manage their time effectively.
- Adaptability . Study modifications, conflicts of interest, budgetary constraints, and other unexpected challenges are common, so research coordinators should be able to adapt to changing circumstances.
Our Online University Degree Programs Start on the First of Every Month, All Year Long
No need to wait for spring or fall semester. It's back-to-school time at WGU year-round. Get started by talking to an Enrollment Counselor today, and you'll be on your way to realizing your dream of a bachelor's or master's degree—sooner than you might think!
Next Start Date {{startdate}}
Interested in Becoming a Clinical Research Coordinator?
Learn more about degree programs that can prepare you for this meaningful career.
The University
For students.
- Student Portal
- Alumni Services
Most Visited Links
- Business Programs
- Student Experience
- Diversity, Equity, and Inclusion
- Student Communities
- Skip to main menu
- Skip to user menu
10 Clinical Research Career Paths
- Industry Features
- General Careers Advice
In 2020, the global Clinical Trials market was estimated at $44.3 billion, and this is expected to grow at an annual rate of 5.7% between 2021 and 2028. The National Institute for Health Research (NIHR) also recorded that between April 2020 and March 2021, 1,390,483 participants took part in Clinical Research across England, which is almost double the numbers from the previous year.
In this article, we look at 10 different career paths within Clinical Research, with an outline of some of the most common responsibilities for each role…
Clinical Trials Manager / Administrator
Clinical Trials Managers / Administrators are responsible for the administrative aspects of clinical trials. Their duties often include:
- Preparing essential documents and ensuring documentation is kept private and confidential.
- Attending safety and study start-up meetings and coordinating investigator meetings.
- Managing clinical trial supplies.
- Reviewing trial protocols and identifying any protocol issues.
- Processing and tracking payments to investigator sites.
More information on the role of a Clinical Trials Manager can be found here.
Clinical Research Associate (CRA)
CRAs are responsible for organising and administering clinical trials and are typically involved in all stages of a trial, from identifying investigator sites to closing down the trial. The responsibilities of a CRA can include:
- Identifying suitable facilities to be used as trial sites and selecting an investigator to be responsible for the site.
- Briefing trial investigators and instructing clinicians on how the trial should be conducted.
- Writing up clinical trial methodologies and designing trial materials.
- Monitoring the progress of clinical trials and preparing final reports.
- Designing and authenticating data collection forms and managing regulatory applications/approvals.
More information on the role of a Clinical Research Associate can be found here.
Clinical Project Manager
Clinical Project Managers are responsible for managing the workers involved in clinical research projects, ensuring protocol compliance whilst coordinating projects to meet clinical objectives. The main responsibilities of a Clinical Project Manager may include:
- Overseeing the enrolment of subjects into clinical trials by assessing the eligibility of potential subjects and tracking the enrolment status of suitable participants.
- Ensuring compliance with protocols and informing investigators of any protocol issues.
- Monitoring study activities to ensure the study remains on schedule and is kept within allocated budgets.
- Maintaining records of study activity, including records of side effect data.
More information on the role of a Clinical Project Manager can be found here.
Pharmacovigilance / Drug Safety Officer
Pharmacovigilance Officers, also known as Drug Safety Officers, are responsible for ensuring that new and existing drugs on the market are safe for patients, and for identifying any issues with these drugs. They may be responsible for:
- Monitoring the effectiveness of new drugs and pharmaceutical products already on the market.
- Monitoring adverse effects to new or existing drugs and flag any early warning signs of these to minimise risk.
- Conducting interviews with patients and healthcare professionals.
- Completing safety update reports and conducting safety audits.
Study Start Up Associate
Study Start Up Associates are integral in making sure that clinical research sites are well prepared to begin a new trial. They can be involved in the following:
- Executing start-up activities before site activation including preparing consent forms, identifying new investigator sites, allocating study budgets, and supporting patient recruitment and retention.
- Ensuring physicians working at research sites are prepared to begin trials.
- Obtaining appropriate ethics and regulatory approvals and ensuring research operations comply with protocols.
- Analysing study start-up metrics to ensure efficiency and identifying areas for development, including in terms of start-up timelines.
More information on the role of a Study Start Up Associate can be found here.
Clinical Research Nurse
Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include:
- Helping to develop new treatments and care pathways for patients.
- Aiding data collection activities.
- Ensuring patients give full consent prior to being enrolled in clinical trials and making sure patients fully understand all aspects of the study before doing so.
- Assisting the principal investigator with pre-study preparation and study start-up activities, including preparing protocols for regulatory and ethical approval, and attending investigator meetings.
- Arranging appointments for potential and enrolled trial participants.
More information on the role of a Clinical Research Nurse can be found here.
Clinical Research Scientist
Clinical Research Scientists are responsible for undertaking medical research in research labs to find more effective ways of diagnosing and curing a variety of illnesses. They may also be responsible for:
- Interacting with patients taking experimental treatments to understand the effectiveness of these treatments and to investigate new ways of improving their wellbeing.
- Working with other medical staff to advise on how to use products and equipment already on or coming to the market.
- Analysing data to further develop treatments and test any new methods of diagnosis and treatment.
Clinical Investigator
Clinical Investigators ensure that the investigation is meeting research expectations and is conducted in line with the investigator statement, investigational plan, and all necessary regulations. By doing so, they protect the welfare of clinical trial participants as well as the integrity of the resulting data. Their responsibilities can include:
- Meeting specific guidelines and/or requirements set by applicable regulatory and ethical bodies.
- Conducting or supervising research to ensure the investigational plan and corresponding study protocols are being followed.
- Notifying relevant bodies of any changes in research activity, including any unanticipated obstacles that may introduce risk to study participants.
- Ensuring informed consent has been obtained from all participants.
- Maintaining records of the clinical studies and preparing reports to be sent to investigation sponsors and other relevant bodies.
Patient Recruitment Specialist
Patient Recruitment Specialists are responsible for recruitment-related activities. Their main responsibilities include:
- Recruiting participants in line with protocol-specific inclusion and exclusion criteria.
- Tracking recruitment progress and developing new and existing recruitment strategies.
- Contacting potential participants to assess eligibility and to schedule site visits.
- Ensure patient information is accurately collected and entered into the relevant database and is protected.
Biostatistician
Biostatisticians provide statistical support to clinical studies and work across all study phases. Typically, their work can include:
- Obtaining clinical data from the Clinical Data Manager to undertake necessary statistical analyses. Interpreting the meanings of statistical outputs resulting from different analyses.
- Assisting the Clinical Trial Manager in writing up the final technical paper for the study, sharing findings from statistical analyses.
- Analysing safety and efficacy data and applying statistical methods to develop the science of data analysis.
More information on the role of a Biostatistician can be found here.
Current Opportunities in Clinical Research…
Take a look at current opportunities in Clinical Research here and set up job alerts to be notified of the latest opportunities in the industry.
* Article updated March 2024
Related links
- Jobs in Clinical Research
- More Careers Advice
Share this article
Related articles

FluoGuide appoints Jens Ellrich as Chief Medical Officer

Stryker announces definitive agreement to acquire Vertos Medical, Inc., expanding interventional pain management solutions

Great Place To Work®: BioPharma Recognised Amongst Top-Ranking Sectors for Employee Wellbeing
Latest articles, about diaceutics, cv6 therapeutics.
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
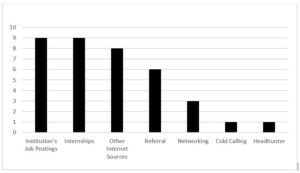
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
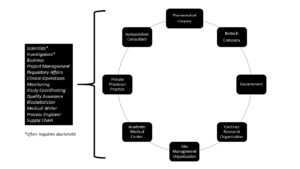
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
| Clinical Research Patient Recruiter | PPD | Bachelor’s degree and related experience |
| Clinical Research Assistant | Duke University | Associate degree |
| Clinical Trials Assistant | Guardian Research Network | Bachelor’s degree and knowledge of clinical trials |
| Clinical Trials Coordinator | Advarra Health Analytics | Bachelor’s degree |
| Clinical Research Specialist | Castle Branch | Bachelor’s degree and six months in a similar role |
| Clinical Research Technician | Rose Research Center, LLC | Knowledge of Good Clinical Practice and experience working with patients |
| Clinical Research Lab Coordinator | Coastal Carolina Research Center | One year of phlebotomy experience |
| Project Specialist | WCG | Bachelor’s degree and six months of related experience |
| Data Coder | WCG | Bachelor’s degree or currently enrolled in an undergraduate program |
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

The Industry Shift Toward Decentralized Clinical Trials: Impacts on Quality Management, Participant Outcomes, and Data Management

Insights into the Clinical Research Associate Career Pathway

The Future of Multi-Omics in Cancer Clinical Trials
- × Search Close Search
Clinical Research Coordinator Jobs: How to Start a Rewarding Career
Actalent article about opportunities available in entry level clinical research jobs.
The fight against cancer needs foot soldiers - lots and lots of them. Heck, it needs warriors.
Cancer research in the U.S. is continuing to drive growth in the need for clinical trials. That's why there's such a high demand for clinical research coordinators (CRCs) to run the clinical trials that test new drugs, treatments and medical devices.
If you're interested in becoming a research coordinator, there are plenty of jobs out there for you. You can work with innovative technology and use the job as a stepping stone to even bigger career opportunities.
"If I were to pick a second career, it would definitely be in clinical research. That's because of the opportunities that are out there right now - not to mention the cool technology and the cures that they're coming up with," said Michael Mazza , a divisional practice lead based in the Philadelphia area. "Just in our office, we consistently have anywhere from three to eight openings for research coordinators at any given time."
We asked Mazza and Lacy Preddie , an account recruiting manager, more questions about this hot career path.
What does a research coordinator do?
CRCs recruit patients and conduct the day-to-day operations for clinical trials. Most coordinators work in research hospitals.
"They are very hands-on, literally enrolling the patients who are going to be in the trial," Preddie said.
Do you need a college degree?
Although it varies by employer, applicants are typically required to have a bachelor's degree in clinical research management, medical technology, public health or nursing.
"Most frequently, candidates have either a science-based background or a background in public health," Preddie said.
How much do clinical research coordinators earn?
In the Philadelphia area, if you're right out of college (with a science-based degree), an entry-level job might pay $18 to $21 an hour. A higher-level coordinator can earn "probably upwards of $65K" annually, Mazza said. Project managers can earn up to $80,000 a year.
Do many people start their careers as clinical research coordinators?
Yes! "This is usually a stepping-stone into clinical research," Preddie explained.
Preddie works with a lot of research coordinators who view the gig as a gateway to a rewarding career. She helps them get on the right path.
"Nobody just falls into coordinating," she said. "They usually have some type of plan in place for where they want to go, or where their interests lie."
Three career paths for CRCs:
1. Become a Clinical Research Associate (CRA)
CRAs fly in to monitor and review how the clinical trial is going, to check the medical data, and to judge the research coordinator's work. And they get paid more.
"They make the big bucks," Mazza said of CRAs. "They're making $70, $80 an hour."
Starting as a coordinator can be a good way to climb into a CRA role. Also, a lot of the larger employers in the clinical research field offer training programs that groom coordinators to take that next step.
2. Go to medical school
Check back next week for a follow-up: Career options for experienced clinical research coordinators.
Looking to take the next step in your career? Visit our job board to search job postings and connect with clinical CRCs. Create a free career account today to customize your search based on your skills and interests. Our recruiters are available to provide advice that you can use and direct you to the right opportunity for you, including those not posted publicly.
Relevant Insights

Learning & Development is at the forefront of company-wide communication in the aftermath of COVID-19. Actalent shares advice on what to do differently.

How to adapt your workforce in a changing environment

More and more pharmaceutical companies are turning to computer-aided tools and methods to assist in discovery efforts, helping to solve complex problems in drug design and development.

Guest Column | November 24, 2020
Starting a career in clinical research: 7 things we wish we knew.
By Laurie Halloran and Michelle Pratt, Halloran Consulting Group, Inc.

This experience has shaped my passion for clinical research education at all levels and my vow to pay it forward. I try to consistently work on initiatives that bring better learning opportunities and more defined competencies and standards to clinical research roles. – Laurie Halloran
In recent years, careers in clinical research have become more plentiful and the opportunities for formal clinical research education are more accessible. The industry, however, is still looking at a talent shortage that could become acute in the next decade. 1 A main contributor to this shortage is a lack of focused intervention at every level to identify the skills, behaviors, and knowledge that will enable motivated candidates to begin and mature their careers in the industry. The real challenge clinical research professionals face at every stage on their career paths is the need to be two things: technically astute (e.g., regulatory knowledge, medical/pathophysiology expertise, data science, technology solution navigation) and emotionally intelligent. These two components of clinical research professionals are pivotal to the success at every level. The intent of this three-part series is to provide insight and guidance to help professional clinical researchers effectively navigate their careers.
Entry-Level Clinical Research Positions And Potential Career Paths
A clinical research coordinator (CRC) 2 works under the direction of a principal investigator (PI) at a clinical site to coordinate and facilitate the daily clinical research activities, including screening and recruiting patients, collecting and recording data, and maintaining study documentation. Working at the site provides a different perspective of clinical research than a CRA or clinical trial associate (CTA) role. Progression on the site side of clinical research typically starts with more senior CRC roles, CRC manager, and, depending on the size of the research site, even leading an entire research department. Many CRCs also move to the industry side of clinical research into a CTA or CRA role.
CTA is a position that supports management of clinical trials under the direction of the clinical trial manager (CTM). In this role, a CTA gains exposure to many aspects of trial management, including working directly with study plans and timelines, vendor oversight, patient enrollment, regulatory documentation, and oversight of the trial master file (TMF) (all study documentation). Many CTAs advance their career though more senior CTA roles, CRA roles, and eventually as CTM or clinical project manager (CPM).
A CRA 3 is the most commonly available clinical research position. In this role, a CRA acts as a liaison between the clinical trial management and the clinical sites that are enrolling subjects. The main responsibilities are clinical monitoring, overseeing the progress of the clinical trial at the site and ensuring that it is being conducted appropriately. The daily focus is on protocol and regulatory compliance, data reliability, and the proper care, treatment, and safety of patients. Many CRA positions can be found at contract research organizations (CROs); however, some sponsor companies also have typical CRA roles or an in-house CRA position. In terms of career development, a CRA typically progresses up through more senior CRA roles to team lead, CRA manager, or potentially to CPM.
Any of these positions is a great way to get into clinical research. Each of these positions provides an education on all elements of clinical research and a foundation for future roles and growth in the industry. By getting this broad view, you will be able to learn more about the specific aspects of clinical research that interest you so you can tailor your career path 4 in that direction. In addition, try to identify an area that continues to challenge you and helps you grow.
If you are interested in exploring different options, we suggest visiting the ACRP’s Find Your Element campaign. 5
Advice From Former Entry-Level Candidates
While the behaviors and skills expected for an entry-level position in clinical research are similar to those that would be expected of any professional candidate, the importance of patient safety, the volatility of the industry, and the number of technical skills you must develop in a short period of time are challenges unique to the life sciences space. For those who have not had any formal training in clinical research, here are seven insights that would have been helpful at the beginning of our careers.
1. Patient Safety Drives All the Work You Do
Clinical research has strict regulations worldwide that have been inspired by key historical events (e.g., Tuskegee syphilis experiments, Jesse Gelsinger’s death). 6,7,8 Change has often come following tragedy or public outcry. Understanding the historical context helps you understand why clinical research is differentiated from other experimentation. Also, it is important to understand that whether or not you are working directly with patients, you can impact patient safety.
2. Understand the “Whys”
You can work in this industry for a long time and never be directly involved in drug or device development. Review the drug discovery and development process 9 to understand the different phases of development and associated regulations. Figure out where your position fits into this process and why it is important. Become familiar with the roles of the different departments in your company and why you need to interact with them. Understanding the bigger picture, emphasizing the reason why every responsibility you have, regardless of career level, is meaningful and important to help you understand why the clinical trial you are working on is being done.
3. Learn your GxPs
Good practice quality guidelines 10 ensure that your drug or device is safe and functions as expected. Having at least a high-level understanding of these guidelines will help you approach your work with a quality mindset and support a culture of quality at your company.
4. If It Is Not Documented, It Didn’t Happen
Everything in clinical research must be documented, and document control is not optional. All companies should have standardized processes for the preparation, recording, and correction of data as well as the maintenance of the records throughout the life cycle of a document. Be organized in your document filing and don’t put your TMF filing and maintenance on the back burner.
5. You Will Have Many Questions
In this industry, you will never have all the answers, regardless of how experienced you are. Questions not only help you but help study team members or sites who “don’t know what they don’t know.” Question things that do not make sense operationally (e.g., ask if a procedure is really necessary when developing a protocol), always considering the patients, the big picture, and your objectives.
6. Communication Skills Are Key 11
With so many moving parts in clinical research, having effective communication habits is necessary. Educational programs do not necessarily set you up with the soft skills 12 that you need when entering the workforce. Become familiar with the concept of soft skills and when you join a company, take the time to understand their communication best practices.
7. You Are the Driver of Your Career
Larger companies in the industry may have a developed training program; however, at many smaller companies you will be learning on the job. Be as proactive as you can to create a network and a system of support (e.g., a coach or mentor). Volunteer for opportunities outside of your comfort zone, mentor others, and learn as much as you can. Advocate for yourself and try not to compare yourself to others, as there are so many different needed skillsets in this industry. Find a path that feels like the right fit, based on your strengths and passions.
As an individual contributor in clinical research, your speed, high-quality results and deliverables, proactivity in keeping management informed, and attention to detail are all equal. Different skills, abilities, and insights will be needed depending on if you would like to continue in a contributing role or move toward management and leadership. In our next installment of this series, we will discuss the transition from an individual contributor to a management role.
References:
- https://www.pharmavoice.com/article/2016-11-talent-war/
- https://forteresearch.com/news/roles-and-responsibilities-of-a-clinical-research-coordinator/
- https://clinicalresearchfastrack.com/clinical-research-associate-career/
- https://tracs.unc.edu/index.php/services/education/careers
- https://careersinclinicalresearch.org/
- https://blog.lillytrialguide.com/clinical-trial-history-regulations-regulatory-guidelines-requirements/
- http://cdn2.hubspot.net/hub/149400/file-410979295-pdf/docs/CRT_Timeline_download.pdf
- https://clinicalcenter.nih.gov/recruit/ethics.html
- https://www.nebiolab.com/drug-discovery-and-development-process/
- https://www.ich.org/page/quality-guidelines
- https://www.indeed.com/career-advice/resumes-cover-letters/communication-skills
- https://www.indeed.com/career-advice/resumes-cover-letters/soft-skills
About The Authors:

Like what you are reading?
Sign up for our free newsletter, newsletter signup.

Get the Reddit app
In the medical field, clinicians treat diseases and injuries one patient at a time. But in public health, we prevent disease and injury. Public health researchers, practitioners and educators work with communities and populations. We identify the causes of disease and disability, and we implement large-scale solutions.
Clinical Research Coordinator
Hi all! Ii m currently in my MPH program and have been thinking of making my end career goal working as a clinical research coordinator.
Is anyone here currently working in this profession and could tell me about your average work day? We're you able to get this position straight out of graduation?
By continuing, you agree to our User Agreement and acknowledge that you understand the Privacy Policy .
Enter the 6-digit code from your authenticator app
You’ve set up two-factor authentication for this account.
Enter a 6-digit backup code
Create your username and password.
Reddit is anonymous, so your username is what you’ll go by here. Choose wisely—because once you get a name, you can’t change it.
Reset your password
Enter your email address or username and we’ll send you a link to reset your password
Check your inbox
An email with a link to reset your password was sent to the email address associated with your account
Choose a Reddit account to continue
- Skip to main menu
- Skip to user menu
Clinical Research Coordinator A/B (Department of Pathology and Laboratory Medicine)

- Clinical Research Coordinator A: Bachelor of Science and 1 to 2 years of experience or equivalent combination of education and experience is required.
- Clinical Research Coordinator B: Bachelor of Science and 2 to 3 years of experience or equivalent combination of education and experience is required.
- Effective communication and writing skills required
- Effective problem solving abilities and sound judgement required
- Strong organizational and time management skills required
- Demonstrated ability to work as part of a team, as well as independently required
- Knowledge of IRB and human research protection regulations preferred
- Practical experience designing and coordinating the day-to-day activities of clinical research projects a preferred
- Experience with Redcap or other tools for organizing study data preferred
- Experience with processing of blood specimens and/or phlebotomy a plus
- A strong passion for empirical research and predictive medicine a plus
- Health, Life, and Flexible Spending Accounts : Penn offers comprehensive medical, prescription, behavioral health, dental, vision, and life insurance benefits to protect you and your family's health and welfare. You can also use flexible spending accounts to pay for eligible health care and dependent care expenses with pre-tax dollars.
- Tuition : Take advantage of Penn's exceptional tuition benefits. You, your spouse, and your dependent children can get tuition assistance here at Penn. Your dependent children are also eligible for tuition assistance at other institutions.
- Retirement: Penn offers generous retirement plans to help you save for your future. Penn's Basic, Matching, and Supplemental retirement plans allow you to save for retirement on a pre-tax or Roth basis. Choose from a wide variety of investment options through TIAA and Vanguard.
- Time Away from Work: Penn provides you with a substantial amount of time away from work during the course of the year. This allows you to relax, take vacations, attend to personal affairs, recover from illness or injury, spend time with family—whatever your personal needs may be.
- Long-Term Care Insurance: In partnership with Genworth Financial, Penn offers faculty and staff (and your eligible family members) long-term care insurance to help you cover some of the costs of long-term care services received at home, in the community or in a nursing facility. If you apply when you're newly hired, you won't have to provide proof of good health or be subject to underwriting requirements. Eligible family members must always provide proof of good health and are subject to underwriting.
- Wellness and Work-life Resources : Penn is committed to supporting our faculty and staff as they balance the competing demands of work and personal life. That's why we offer a wide variety of programs and resources to help you care for your health, your family, and your work-life balance.
- Professional and Personal Development: Penn provides an array of resources to help you advance yourself personally and professionally.
- University Resources: As a member of the Penn community, you have access to a wide range of University resources as well as cultural and recreational activities. Take advantage of the University's libraries and athletic facilities, or visit our arboretum and art galleries. There's always something going on at Penn, whether it's a new exhibit at the Penn Museum, the latest music or theater presentation at the Annenberg Center, or the Penn Relays at Franklin Field to name just a few examples. As a member of the Penn community, you're right in the middle of the excitement—and you and your family can enjoy many of these activities for free.
- Discounts and Special Services : From arts and entertainment to transportation and mortgages, you'll find great deals for University faculty and staff. Not only do Penn arts and cultural centers and museums offer free and discounted admission and memberships to faculty and staff. You can also enjoy substantial savings on other goods and services such as new cars from Ford and General Motors, cellular phone service plans, movie tickets, and admission to theme parks.
- Flexible Work Hours: Flexible work options offer creative approaches for completing work while promoting balance between work and personal commitments. These approaches involve use of non-traditional work hours, locations, and/or job structures.
- Penn Home Ownership Services: Penn offers a forgivable loan for eligible employees interested in buying a home or currently residing in West Philadelphia, which can be used for closing costs or home improvements.
- Adoption Assistance: Penn will reimburse eligible employees on qualified expenses in connection with the legal adoption of an eligible child, such as travel or court fees, for up to two adoptions in your household.
Share this job
Get job alerts
Create a job alert and receive personalized job recommendations straight to your inbox.
Clinical Research Coordinator
Job posting for clinical research coordinator at community memorial healthcare, compensation.
Salary Range: $34.56 - $48.11 / hour
The pay range above represents the lowest possible rate for the position and the highest possible rate. Factors that may be used to determine where newly hired employees will be placed in the pay range include the employee specific skills and qualifications, relevant years of experience and comparison to other employees already in this role. Most often, a newly hired employee will be placed below the midpoint of the range.
If you are viewing this posting on a job site, please visit our company page and search for the opportunity to view the pay range: https://careers-mycmh.icims.com/jobs
Responsibilities
Position Overview:
Coordination of a variety of research projects from pre-study implementation through study closure. The incumbent independently manages all aspects of multiple clinical trials or other research projects as assigned, including but not limited to: research participant recruitment, screening and enrollment; completion of protocol required visits and procedures; collection and reporting of data; coordination of and participation in sponsor monitoring visits or federal audits; query resolution; adverse event reporting, source documentation and research record development and maintenance; study drug accountability; specimen collection, processing and shipment. The incumbent ensures compliance of the research program and its studies with all applicable federal, state, regulatory agency, and institutional requirements.
Qualifications
- High School Diploma
- Current certification (CCRP or CCRC) by the Society of Clinical Research Associates (SOCRA) or Association of Clinical Research Professionals (ACRP) or required within one year of hire
- Current certificate of Human Subjects Protection and Good Clinical Practice training
- Minimum of two (2) years experience conducting clinical trials
- Thorough knowledge and understanding of research regulatory requirements involving human subjects’ research, including FDA, OHRP and GCP requirements
- Experience in implementation of research protocols and clinical trials processes
- Experience in preparing and maintaining regulatory documents and other IRB-related study documentation
- Lab processing experience
Preferred:
- Bachelor's degree in a science or health-related field
- Worked as primary research coordinator for at least 5 drug or device trials
Community Memorial Health System was established in 2005 when Community Memorial Hospital in Ventura merged with Ojai Valley Community Hospital. It is comprised of these two hospitals along with a network of primary and specialty care health centers serving various communities across west Ventura County. Our health system is a community-owned, not-for-profit organization. As such, we are not backed by a corporate or government entity, nor do we answer to shareholders. We depend on – and answer to – the communities we serve.
Community Memorial Health System Benefits
To help heal, comfort, and promote health for the communities we serve, Community Memorial Health System (CMHS) takes care of our community of employees so our local community can be cared for. That’s why we provide competitive benefits, along with great career choices, training, and leadership development. Our total rewards package provides benefits that support you and your family’s health and wellness in all aspects of life. From our top tier insurance plans to our employee assistance program, take advantage of what CMHS has to offer so you and your loved ones can have peace of mind now and for years to come. CMHS is here for you and your family every step of the way.
- Competitive Pay
- Shift Differentials (Nights & Weekends)
- In-House Registry Rates
- Fidelity 403(b) Retirement Plan
- Paid Time Off
- Medical (EPO/PPO), Dental, & Vision Insurance Coverage
- Voluntary Worksite Benefits
- Employee Assistance Program Available 24/7 (EAP)
- Tuition Reimbursement
- Public Service Loan Forgiveness (PSLF)
- Recognition programs
- Employee service recognition events
- Home, Retail, Travel & Entertainment Discounts
- National Hospital Week and National Nurses Week celebrations
“We are an AA/EEO/Veterans/Disabled Employer”
Salary : $35 - $48
Apply for this job
Receive alerts for other Clinical Research Coordinator job openings
Report this Job
Sign up to receive alerts about other jobs that are on the Clinical Research Coordinator career path.
Click the checkbox next to the jobs that you are interested in.
Sign up to receive alerts about other jobs with skills like those required for the Clinical Research Coordinator .
Clinical Data Analysis Skill
- Clinical Data Specialist Income Estimation: $60,766 - $86,189
- Health Value Analyst Income Estimation: $63,978 - $112,072
Clinical Research Skill
- Clinical Research Associate II Income Estimation: $69,015 - $91,158
- Clinical Outcomes Analyst II Income Estimation: $75,704 - $93,896
Job openings at Community Memorial Healthcare
Not the job you're looking for here are some other clinical research coordinator jobs in the ventura, ca area that may be a better fit., we don't have any other clinical research coordinator jobs in the ventura, ca area right now..
Assistant Clinical Research Coordinator
Om Research LLC , Camarillo, CA
Clinical Research Coordinator II
Care Access , Thousand Oaks, CA

Research Coordinator
- Madison, Wisconsin
- SCHOOL OF MEDICINE AND PUBLIC HEALTH/RADIOLOGY-GEN
- Staff-Full Time
- Opening at: Aug 23 2024 at 15:35 CDT
- Closing at: Sep 8 2024 at 23:55 CDT
Job Summary:
The Department of Radiology, University of Wisconsin - Madison, School of Medicine & Public Health is seeking a Research Coordinator who will serve as data steward for radiomics (machine learning in radiology imaging) projects ongoing in Dr. Tiwari's lab. The research conducted by Tiwari lab utilizes substantial amounts of medical imaging and clinical data to build and optimize AI and machine learning models that identify computerized image-based phenotypes, and their associations with genomics and histo-pathology for disease characterization, with the aim of developing personalized diagnostic tools towards improved early diagnosis, prognosis, and response to treatment for neurological conditions and other diseases. The Research Coordinator will be responsible for being the point person regarding data acquiring, deidentification, clinical data management, and database upkeep of an ever-growing repository of medical imaging and clinical information for a variety of diseases (e.g., brain tumors, stroke, Alzheimer's, MS, breast cancer, pancreatic cancer, and liver disease), as well as coordinating data-related efforts across different multi-site collaborations. Tiwari lab utilizes medical imaging datasets from a variety of sources, including from the UW Health Electronic Health Record, through data user agreements with collaborating institutions, and publicly available sources. Under the guidance of the PI and lab manager, this role will manage identifying and downloading new datasets, perform quality control and reporting on new and existing datasets, and preprocessing and annotating images to ensure high-quality data is fed into the AI and machine learning models enabling this research.
Responsibilities:
- 40% Assists with conducting experiments or interviews, collecting and analyzing data, and documenting results according to established protocols
- 10% Assists in composing and organizing research reports and manuscripts to provide updates on unit objectives
- 30% Maintains organization of research records according to established policies and procedures
- 10% Quality control of the images for AI analysis using approaches developed by the lab in detecting artifacts, image quality
- 10% Annotate regions of interest on MRI scans for AI analysis. Segment regions of interest i.e. Cancer, disease, inflammation, notable pathologies on radiographic scans for AI analysis
Institutional Statement on Diversity:
Diversity is a source of strength, creativity, and innovation for UW-Madison. We value the contributions of each person and respect the profound ways their identity, culture, background, experience, status, abilities, and opinion enrich the university community. We commit ourselves to the pursuit of excellence in teaching, research, outreach, and diversity as inextricably linked goals. The University of Wisconsin-Madison fulfills its public mission by creating a welcoming and inclusive community for people from every background - people who as students, faculty, and staff serve Wisconsin and the world. For more information on diversity and inclusion on campus, please visit: Diversity and Inclusion
Required Bachelor's Degree Medical Imaging, Data Science, Biomedical Engineering, or a related field
Qualifications:
-At least 1-2 years of related experience in medicine/radiology/medical imaging techniques is preferred. -Professional and effective oral and written communications skills. Must be able to clearly relay information to faculty PI's and other researchers and prepare reports as needed. -Effective data management and organizational skills; ability to train, work with, and elicit cooperation from team members with regards to data stewardship. -Ability to problem-solve effectively; ability to develop and implement alternative solutions as needed. -Ability to learn and apply new ideas, techniques, processes, policies, functions, etc. Must be highly motivated and a self-starter. -Familiarity with image scanners and scanning protocols is preferred.
Work Schedule:
Monday -Friday, 7:30AM -4:30PM
Full Time: 100%
Appointment Type, Duration:
Ongoing/Renewable
Minimum $19.83 HOURLY Depending on Qualifications
How to Apply:
To apply for this position, please click on the "Apply Now" button. You will be asked to upload a resume, cover letter, and list of three professional/supervisor references as a part of the application process. References will not be contacted without prior notice. Please ensure that the resume and cover letter address how you meet the minimum/preferred qualifications for the position.
Alex Scaffidi [email protected] 608-262-7480 Relay Access (WTRS): 7-1-1. See RELAY_SERVICE for further information.
Official Title:
Research Coordinator(RE034)
Department(s):
A53-MEDICAL SCHOOL/RADIOLOGY/RADIOLOGY
Employment Class:
University Staff-Ongoing
Job Number:
The university of wisconsin-madison is an equal opportunity and affirmative action employer..
You will be redirected to the application to launch your career momentarily. Thank you!
Frequently Asked Questions
Applicant Tutorial
Disability Accommodations
Pay Transparency Policy Statement
Refer a Friend
You've sent this job to a friend!
Website feedback, questions or accessibility issues: [email protected] .
Learn more about accessibility at UW–Madison .
© 2016–2024 Board of Regents of the University of Wisconsin System • Privacy Statement
Before You Go..
Would you like to sign-up for job alerts.
Thank you for subscribing to UW–Madison job alerts!

COMMENTS
Search 1000s of Clinical Research Coordinator Jobs. New Full Time & Part Time Jobs. Clinical Research Coordinator Jobs Open For Immediate Hire - Search & Apply Online Now!
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private ...
This series also provides employees with a clear path for career progression within Michigan Medicine. The following are market titles in this career ladder: Clinical Research Assistant ( 103921) Clinical Research Technician ( 103922) Clinical Research Coordinator Associate ( 103923) Clinical Research Coordinator Intermediate ( 103924)
Before the author was a CRA, he was a research coordinator for fourteen years. This article describes how the author made the transition from clinical research coordinator to CRA/clinical research monitor and includes some suggestions for those looking to make a similar career change. When to Transition from Research Coordinator to CRA
Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities. 1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol adherence.
Instead, they play an essential role in overseeing new methods for medical treatment. Clinical research coordinators typically complete the following tasks as part of their job: Oversees administrative duties related to research practices. Works with the principal investigator to determine clinical research procedures.
Here is a guide on how to pursue a career as a CRC: Obtain a Relevant Bachelor's Degree: Most CRC positions require a bachelor's degree in a relevant field such as biology, chemistry, nursing, psychology, or healthcare administration. Some positions might prefer candidates with a degree in a specific area related to the type of clinical ...
Salaries for clinical research coordinators vary based on factors such as experience level, geographical location, and industry. Entry-level clinical research coordinators can expect a salary range of $45,000 to $60,000 annually. Mid-level coordinators typically earn between $60,000 and $80,000 per year.
Here are the key steps to guide you on your journey: Bachelor's Degree: Obtain a bachelor's degree, preferably a Bachelor of Science in a relevant subject such as clinical research administration, health sciences, public health, microbiology, or a related field. Work Experience: Gain practical experience in healthcare or clinical research.
Clinical research career progression paths can vary depending on one's interests, the organization they work for, and specializations. Advancement opportunities include progressing from a clinical research coordinator (CRC) to a senior CRC or clinical research associate (CRA), from a CRA to a senior CRA or clinical trial manager, or from a ...
The typical day of a clinical research associate includes planning and managing clinical research projects for pharmaceutical companies. They may recruit participants, coordinate schedules, input data, and oversee trials. In their career, clinical researchers may also be in charge of ensuring that researchers follow all local and federal ...
2. Earn a bachelor's degree. Prepare for a clinical research coordinator career by studying for a bachelor of science degree in health science, biological sciences, life research, medical technology or clinical research. This degree typically takes four years to complete. 3.
A Clinical Research Coordinator (CRC) plays a vital role in this field, ensuring research is conducted ethically and efficiently. If this sounds like the path for you, here are 7 essential steps to becoming a successful CRC: Earn a Relevant Degree: A bachelor's degree in a science-related field like biology, chemistry, or healthcare ...
The educational requirements for a clinical research coordinator position can differ based on the organization and job responsibilities. Employers typically seek candidates with at least a bachelor's degree in a science or medical field. Many clinical research coordinators have educational backgrounds in public health, biology, health science ...
Clinical Research Nurse. Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include: Helping to develop new treatments and care pathways for patients. Aiding data collection activities.
A Clinical Research Coordinator (CRC) is responsible for the following tasks: Recruiting and screening participants: Identifying potential participants for the trial, contacting them to explain the study, and assessing their eligibility to participate. Obtaining informed consent: Explaining the study to participants and ensuring that they ...
Cellular Therapy Research RN Coordinator - BSN 340929 Phoenix, Arizona. Associate Clinical Research Coordinator - Cancer Center 335870 Jacksonville, Florida. Associate Clinical Research Coordinator - Cardiovascular Diseases 340384 Rochester, Minnesota. Clinical Research Coordinator - Comprehensive Cancer Center 331289 Rochester, Minnesota.
After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate). The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry.
December 2020. Melissa Lopez, Clinical Research Supervisor in the Early Phase Investigational Therapeutics Department at HDFCCC, discusses her career path to...
Three career paths for CRCs: 1. Become a Clinical Research Associate (CRA) CRAs fly in to monitor and review how the clinical trial is going, to check the medical data, and to judge the research coordinator's work. And they get paid more. "They make the big bucks," Mazza said of CRAs. "They're making $70, $80 an hour."
Entry-Level Clinical Research Positions And Potential Career Paths. A clinical research coordinator (CRC) 2 works under the direction of a principal investigator (PI) at a clinical site to coordinate and facilitate the daily clinical research activities, including screening and recruiting patients, collecting and recording data, and maintaining ...
The career path for a clinical research coordinator includes jobs as a clinical research professional, a clinical research coordinator, and a clinical trial associate.Clinical research coordinators hold a bachelor's degree in public health or a related field, and some companies may require additional schooling, such as a master's degree.Many clinical research coordinators (CRC) begin working ...
Clinical research coordinator jobs are very entry level with low pay. You can get in with little to no experience but with a bachelor degree in the sciences. Don't make that your end goal. Try getting a CRC position now while in school and then upon graduating move up to manager position. bebepls420.
It typically takes 4-6 years to become a clinical research coordinator: Years 1-4: Obtaining a Bachelor's degree in a relevant field, such as biology, chemistry, or nursing. During this time, students will learn about scientific research methods, medical terminology, and ethical principles in research.
If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440. Job offers
The Clinical Research Coordinator Associate will be responsible for the overall implementation of an assigned set of research protocols assuring efficiency and regulatory compliance. Other responsibilities will include recruiting, screening, assisting in the informed consent process and enrolling subjects in accordance with good clinical ...
Job Type: Officer of Administration Bargaining Unit: Regular/Temporary: Regular End Date if Temporary: Hours Per Week: 35 Standard Work Schedule: Building: Salary Range: $62,400 - $65,000 The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education, licenses, specialty, and ...
Clinical Research Coordinator A/B (Department of Pathology and Laboratory Medicine) University Overview The University of Pennsylvania, the largest private employer in Philadelphia, is a world-renowned leader in education, research, and innovation.
Research salary, company info, career paths, and top skills for Clinical Research Coordinator Apply for the Job in Clinical Research Coordinator at Ventura, CA. View the job description, responsibilities and qualifications for this position.
Many companies seek communications coordinators to promote their brand, so this is a sought-after job. One of the benefits of working as a communications coordinator is that much of the work can be completed at home. However, some might need to travel to oversee certain promotional events.
Job Summary: The Department of Radiology, University of Wisconsin - Madison, School of Medicine & Public Health is seeking a Research Coordinator who will serve as data steward for radiomics (machine learning in radiology imaging) projects ongoing in Dr. Tiwari's lab. The research conducted by Tiwari lab utilizes substantial amounts of medical imaging and clinical data to build and optimize AI ...