- For Teachers

- Everyday Activities
- Experiments

Reaction Time
How fast does your brain send messages to your body?
Ready to test your reaction time?
How fast does the eye send messages to the brain and the brain send messages to your hand muscles to react in time to catch a falling object? Find out using the ruler test!
Watch the video on YouTube: https://youtu.be/p4BkFVCnNe4
You Will Need
At least one friend or family member to be the test subject
Reaction time chart
Reaction time graph
Pen or pencil
Materials & Directions PDF
The reaction time chart and graph are available in the Materials & Directions PDF if you have not already downloaded it.
- Ask student to create a testable question (a hypothesis). Example: Do people of different ages have different reaction times?
- Stand over the test subject with your arm stretched out, holding the ruler with your thumb and forefinger. Put the beginning (end that starts with zero) of the ruler right between the test subject's open fingers.
- Without telling the test subject that you're going to do it, drop the ruler and the test subject catches it as quickly as they can between their fingers.
- Measure the distance on the ruler by recording where the test subject grabbed it.
- Using the reaction time chart, convert the distance to reaction time.
| Distance on Ruler | Reaction Time |
|---|---|
| 5 centimeters | .10 seconds |
| 10 centimeters | .14 seconds |
| 15 centimeters | .18 seconds |
| 20 centimeters | .20 seconds |
| 25 centimeters | .23 seconds |
| 30 centimeters | .25 seconds |
- Use the Reaction time graph to graph your data to measure trends.
- Change different variables to see what might cause reaction time to be faster or slower.
Discovery Questions
Beginning the experiment, during the experiment, after the experiment, how it works.
Reaction time is the time between any kind of event and the response it elicits in a system. The brain is an essential part of developing a quick reaction time.
In this experiment, the eye sees that the ruler has been dropped. This information travels from sensory neurons along the optic nerve from the eye to the brain. The brain processes this information, then sends a signal through motor neurons down the arm to tell the muscles in the hand to close and catch the ruler.
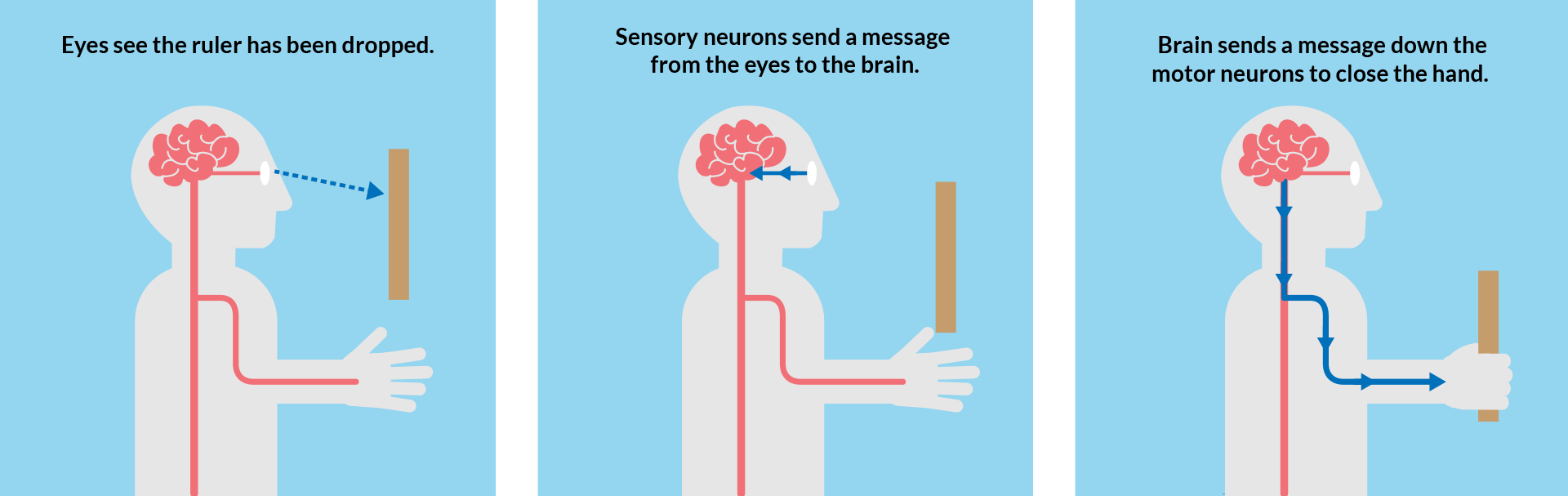
The amount of time this all takes is what makes up our reaction time. Your reaction time depends on your eyesight and the speed that the signals take to travel from your brain to your muscles.
It is possible to improve your reaction time via practice. When we begin to acquire a new physical skill through repetition, our nervous system creates new neural pathways. The more we practice something, the more the members of that neural pathway (eye, brain, muscles) become more well-connected and efficient. This is often referred to as muscle memory.
Simple and choice reaction time tasks

Introduction
In pictures, do it yourself.
In cognitive experimental psychology, we distinguish between simple and choice response time tasks. These two terms are being used in many books papers about cognitive psychology. This lesson explains and demonstrates what we mean with simple and choice response time tasks.
| Type | Definition | Examples |
|---|---|---|
Simple Response Time task (SRT) | There is just one stimulus, and when it appears, you need to respond with the one response there is in this type of experiment | Every time you see a light go on, you need to press the space bar of your computer keyboard. Or the athlete starting to run when the starting gun goes off. |
Choice Response Time task (CRT) | There are multiple stimuli, and each stimulus requires a different response | You will see one of 10 letters presented. Each time you see the letter, you need to press the corresponding letter key of your keyboard. |
People (and animals) can respond a lot faster when there is just one stimulus and one response type (Simple Response Time task). Also, the more stimuli and responses there are, the slower you get (this is known as Hick’s law ).
| Generally speaking, when there is just one stimulus and one response, many people can respond well below 200 ms, that is less than 1/5th of a second! In choice response time tasks with 2 stimuli and 2 responses (that is the simplest possible choice response time task), responding within 250 ms is probably the fastest you can do, but more typically people have an average response somewhere between 350 and 450 ms. Again, a multitude of factors can influence this, including the exact type of stimulus and response mode. |
It is now well established that a person’s response speed is influenced by age and general intelligence (e.g., Deary, Liewald, and Nissan, 2011 ). It is important to note that many other factors play a role as well, for example the conditions under which you perform the task (are you fit or tired, are you hungry, etc). Also, your speed depends on how accurate you aim to be. If you do not want to make mistakes, you will become slower. This is known as the speed-accuracy trade off (this goes back to the work of Woodworth, 1899 ; for a good review see Heitz, 2014 ).
It is important to understand that response times play a crucial role in experimental cognitive psychology. The basic idea is that response times reflect the time it takes to interpret a stimulus, get information from memory, initiate a muscle response, etc. Thus, response times can be used to find out how long basic thought processes take. This idea goes back to the work of the early experiment psychologists in the second half of the 19th century (when the term "cognitive psychology" did not even exist). One of the leading figures in this area of research was the Dutch ophthalmologist Franciscus Donders .
Below you see an example of the simple and the choice response time paradigm.
In the simple reaction time task, you need to wait until you see a black cross on the white square. When that happens, you press as soon as you can the space bar. Thus, there is one stimulus (black cross) and one response (pressing the space bar).

In the choice reaction time task, you need to wait until you see a black cross on one of the four white squares (e.g., there are four different black cross position, which counts as four different stimuli). When that happens, you press as soon as you can the corresponding key (z, x, . or ,). Thus, there are four stimulus-response associations. In this example trial, you need to press the "x" key.

In the demonstration below, you will do both a simple response time task (20 trials) and a choice response time task (20 trials). At the end, you will see your average response speed for the simple and choice reaction time task. You will be slower in the choice reaction time task.
Here we use the Deary-Liewald paradigm, which uses both a simple and a 4-choice response time task. There are two differences:
In the original Deary-Liewald paradigm, there are training blocks and more trials than in this demo.
In the original Deary-Liewald PC version, the keys of the keyboard chosen are great for UK and US keyboards, but not for German and French keyboards. This implementation uses x, c, b, and n because that works on UK, US, German, French keyboards, and probably cover most keyboards around the world.
If you want to do the full version of the Deary-Liewald paradigm, visit the experiment library .
| The time between stimuli varies (randomly) between 1 and 3 seconds. This is an important part of the paradigm. If this random variation would not be used, the simple choice reaction time task would be very simple, because you could predict when the stimulus would appear. |
Click here to try the experiment
Deary, Liewald & Nissan (2011). A free, easy-to-use, computer-based simple and four-choice reaction time programme: The Deary-Liewald reaction time task. Behaviour Research Methods, 43 , 258-268.
Heitz, R. P. (2014). The speed-accuracy tradeoff: history, physiology, methodology, and behavior. Frontiers in Neuroscience, 8 , 1-19.
Woodworth, R. (1899). The accuracy of voluntary movement. Psychological Review, 3 , 1-106
- Earth Science
- Physics & Engineering
- Science Kits
- Microscopes
- Science Curriculum and Kits
- About Home Science Tools
Science Projects > Physics & Engineering Projects > Reaction Time Science Project
Reaction Time Science Project
For Olympic runners and swimmers, a fraction of a second is often the difference between winning a gold medal or a bronze!
Indeed, it’s the distance between winning any medal or returning home with nothing but hopes at another chance in four more years.
And while its impact is most dramatic in running events, speed isn’t only a matter of crossing the finish line first.
In sports, reaction time, the interval between stimulation and reaction, often determines who wins and who loses. Even more importantly, in real-life situations, like when driving a car, it can mean the difference between life and death.
Measure your reaction time with the following project.
What You Need:
- Meter stick
- Dollar bill (optional)
- Flat, sturdy surface, like a tabletop or desktop
- One or more partners
- Reaction time table PDF
What You Do:
1. Have your partner sit or stand with their arm on the flat surface so their wrist extends beyond the edge.

2. Hold the meter stick vertically above your partner’s hand, with the “0” end of the stick just above their thumb and forefinger, but not touching them.
3. Instruct your partner to catch it as quickly as possible as soon as they see it begin to fall.
4. Without warning your partner, drop the meter stick.

5. Record how far it fell before your partner caught it. Consult the reaction time table to determine reaction time. Repeat at least two more times.
6. Switch places with your partner and repeat.
What Happened:
In this experiment, your reaction time is how long it takes your eyes to tell your brain that the meter stick is falling and how long it takes your brain to tell your fingers to catch it. We can use the distance the meter stick fell before you caught it to figure out your reaction time. The following formula is the basis: d = 1/2 gt 2 .
In this formula, “d” equals the distance the object fell, “g” equals gravitational acceleration (9.8 m/s 2 ), and “t” is the time the object was falling. To simplify the process, we’ve provided a reaction time table with the calculations already done.
Try it again with a dollar bill, only start with the bill halfway between the catcher’s thumb and pointer finger. If you’re really brave, you can up the ante and allow whoever catches the dollar bill to keep it. Unless someone anticipates the dollar bill being dropped, the 6-inch bill should fall completely through the catcher’s fingers before the typical human reaction time (about 1/4 second) allows them to catch it.
For further study:
- Talk about what sports depend on having a fast reaction time. How about real-life situations?
- Try the experiment on a variety of people of different ages. Whose reaction time is faster? Boys or girls? Adults or kids?
- Repeat the experiment, only this time, have the catcher whistling throughout. Did that make reaction time faster, slower, or the same?
- Can you improve your reaction time by repeating the experiment several times daily? Practice for a week then test yourself again to see.
More Sensory Projects:
- Eye Chart Vision Test
- Two-point Discrimination
- Using the Five Senses
Physics & Engineering
Welcome! Read other Physics & Engineering related articles or explore our Resource Center, which consists of hundreds of free science articles!
Shop for Physics Supplies!
Home Science Tools offers a wide variety of Physics products and kits. Find physics & engineering tools, equipment, STEM kits & more for kids and adults.
Related Articles

Homopolar Motor – Make a Spinning Wire Sculpture
In this experiment, we will make a homopolar motor! To make a simple motor (homopolar motor) that doubles as a work of art you will need three things – a battery, magnet, and wire. Use one of our neodymium magnets to power the spinning wire motor. What You Will Need:...

Solar Energy Matching Game
Print out this page on a sheet of heavy paper or cardstock. Kids can color the pictures and cut out the squares to make a matching game. Half of the squares show a way to use solar energy as an alternative to the picture shown on the other squares. Place all the...

Simple Spring Break Science Projects
Spring break is here! What will you do with your time off? Perhaps you're looking forward to a family vacation, or a few days of down time at home. Either way, find a quick and easy project that's sure to put a sparkle in the eye of any science lover, or win over a...

Sink or Float Worksheet
Use the Sink or Float Worksheet with the "Sink or Float?" science project to encourage kids to make predictions, perform tests, and record their results. This project is perfect for indoor discovery - especially in colder months! Put a towel under a large container of...

Physics Science Fair Projects
Find physics science fair project ideas about magnetism, electricity, energy and solar power, and more.
JOIN OUR COMMUNITY
Get project ideas and special offers delivered to your inbox.

Neuroscience for Everyone!
- Experiments
Experiment: How Fast Your Brain Reacts To Stimuli
How fast do you think you are? Do you know what a reflex and a reaction are? This lesson plan tells all about the quickness of your nervous system and the muscular system, which the nervous system innervates.
What will you learn?
In this experiment you are going to be introduced to what a reflex and reaction are and how we go about measuring them. Do not worry we won't be throwing soccer balls at your face. . . yet!
Prerequisite Labs
Note: Backyard Brains has released a digital reaction timer that uses your body's electrical signals to measure your reaction time! If you enjoy this experiment and want to take it to the next level, check out the Backyard Brains Reaction Timer !
The speed of your reactions play a large part in your everyday life. Fast reaction times can produce big rewards, for example, like saving a blistering soccer ball from entering the goal. Slow reaction times may come with consequences.

Reaction time is a measure of the quickness an organism responds to some sort of stimulus. You also have "reflexes" too. Reflexes and reactions, while seeming similar, are quite different. Reflexes are involuntary, used to protect the body, and are faster than a reaction. Reflexes are usually a negative feedback loop and act to help return the body to its normal functioning stability, or homeostasis. The classic example of a reflex is one you have seen at your doctor's office: the patellar reflex.

This reflex is called a stretch reflex and is initiated by tapping the tendon below the patella, or kneecap. It was first independently described in 1875 by two German neurologists, Wilhelm Heinrich Erb and Carl Friedrich Otto Westphal. In their original papers Erb referred to the reflex as the "Patellarsehnenreflex" while Westphal denoted it as the "Unterschenkelphanomen". Thankfully, we now refer to it as the patellar reflex.
This reflex is also known as a "reflex arc". It is a negative feedback circuit that is comprised of three main components:

Quick! We're timing you...
The knee reflex arc is a spinal reflex, and the circuit is drawn above. This picture shows how the sensory (afferent) neuron sends information through the dorsal root ganglion into the spinal cord; where the signal splits into two different paths. The first is the motor neuron (efferent) leading back to the quadriceps. When your quad muscle's motor neuron receives the information it fires and causes your lower leg to spring forward up in the air. The second signal from the sensory neuron travels to an interneuron which sends a signal to the motor neuron (efferent) leading to the hamstring. This signal tells your hamstring to relax so there is no negative force acting on the quadriceps muscle when it contracts. Both signals work together and all of this happens in the spinal cord without going to the brain. It never needs the brain.
You may be asking how a knee reflex arc and a soccer player dealing with an oncoming ball are different. Are both not reflexes? While it may seem that a soccer player negotiating an oncoming ball is a simple fast reflex, it is actually a symphony of hundreds of thousands of neurons working together to produce a conscious decision. Does the player catch, dodge, or bat away the ball? This choice is what makes a reaction.

When a soccer player realizes the ball is blistering towards him, there is visual information that has to be processed and decisions regarding a correct course of action. The brain then needs to send many signals to various muscles. Feet begin to move, hands might travel in front of the face, and eyes may close shut, along with many more processes. This is the work of many neurons as well as numerous systems and circuits in the brain, and what's more, and you can train and enhance your skill through practice. This is how you get better at sports over time.
Like all science, the history of the reaction time discovery is peculiar. Dutch physiologist F.C. Donders in 1865 began to think about human reaction time and if it was measurable. Prior to his studies scientists thought that human mental processes were too fast to be measured. This assumption was proved incorrect with the help of Charles Wheatstone, an English scientist and inventor. In 1840 Wheatstone invented a device, much like his early telegraph system invention, that recorded the velocity of artillery shells. Donders used that device to measure the time it took from when a shock occurred on a patient's foot until when that patient pressed a button. The button had to be pressed by the left or right hand matching the left or right foot that was shocked. His study tested 2 conditions: in the first, the patient knew in advance which foot was to be shocked; in the other condition, the patient did not know. Donders discovered a 1/15 second delay between patients who knew which foot was to be shocked versus patients that did not know. Notably, this was the first account of the human mind being measured!

These efforts continue today, with the improvement of "non-invasive" imaging technologies like fMRI, PET, EEG, etc... You may have had one of these scans in the hospital.

How quickly neurons move information is called the "speed of neural transmission"; we studied it in experiment 11 when we measured the conduction speed of axons in earthworms. This is only one of the speed bottlenecks though. You also have to deal with the synapse (which we studied in experiment 8). Furthermore, the quickness of reaction times can differ depending upon what type of stimulus you are reacting to and what kind of task you are doing.
In this experiment you and a friend will be testing each other's reaction times using a simple 12 inch ruler. You will be testing not only visual stimulus, but also auditory and tactile stimuli.
This experiment will be broken into two phases. The first test will use one ruler, while the second test will use two.
Experiment 1: In this phase you and your partner will test visual, auditory, and tactile reaction times using one ruler.

Here is the table for the first experiment:

Experiment 2: In this phase you and your partner will test visual and auditory reaction times using two rulers.
Here is the table for the second experiment:

In your chart above you are going to take all the centimeter measurements you have collected and convert the measurement in centimeters to seconds. This will tell you how long it takes, in seconds, an object (the ruler) to fall a certain distance. The formula below is comprised of three variables.

Here is an example of the equation being used:

It may seem tedious to convert by hand each number you recorded so instead you will be provided with a quick chart to convert your centimeter measurement to seconds. However, there are several values missing in the table. You will need to fill them out to complete the table. Use the equation above to fill out the remainder of the chart. If you are savvy you can also design a computer program to do this.

After using the chart and converting your centimeter measurements into seconds you will have your ruler reaction time in seconds. Looking at your data you might be thinking how you compare to the human average reaction time. Here it is! The average reaction time for humans is 0.25 seconds to a visual stimulus, 0.17 for an audio stimulus, and 0.15 seconds for a touch stimulus.
Concise Handout for the Classroom
Science fair project ideas.
May 24, 2012
Speedy Science: How Fast Can You React?
A swift science activity from Scientific American
By Daisy Yuhas
Key concepts: Reaction time Neuroscience Gravity Introduction Think fast! Have you ever noticed that when someone unexpectedly tosses a softball at you, you need a little time before you can move to catch it (or duck)? That's because when your eyes see an incoming signal such as a softball, your brain needs to first process what's happening—and then you can take action. In this activity, you can measure just how long it takes for you to react, and compare reaction times with your friends and family. Background You may not realize it, but when your senses pick up clues from the outside world—the smell of baking cookies, the color of a stoplight, the rrrring! of an alarm clock—it takes a fraction of a second for you to recognize that signal and respond. During that time your brain receives information from your senses, identifies a possible source, and allows you to take action. The jam-packed fraction of a second is called your reaction time. This activity teaches you about your brain's reaction time, but it also relies on the laws of physics. Specifically, you can calculate your reaction time using our handy chart, which is based on how quickly a ruler falls. How do we know how quickly your ruler will fall? Gravity pulls all objects toward Earth's center at the same speed. If you want to try this out at home, try dropping a tennis ball and a basketball from the same height: They should both hit the ground at the same time! Materials · Ruler (inches or metric) · Paper · Pencil · Chart (below)
(inches | centimeters) | (seconds | milliseconds) | ||
|
|
|
|
4 in. | 10 cm. | 0.14 sec. | 140 ms. |
6 in. | 15 cm. | 0.17 sec. | 170 ms. |
8 in. | 20 cm. | 0.2 sec. | 200 ms. |
10 in. | 25.5 cm. | 0.23 sec. | 230 ms. |
12 in. | 30.5 cm. | 0.25 sec. | 230 ms. |
17 in. | 43 cm. | 0.3 sec. | 300 ms. |
24 in. | 61 cm. | 0.35 sec. | 350 ms. |
31 in. | 79 cm. | 0.4 sec. | 400 ms. |
39 in. | 99 cm. | 0.45 sec. | 450 ms. |
48 in. | 123 cm. | 0.5 sec. | 500 ms. |
69 in. | 175 cm. | 0.6 sec. | 600 ms. |
Preparation • You need to use some math skills in this challenge. To make things easier, we've provided a chart, above, that you can print or copy out on a piece of paper. The basic rule: 100 milliseconds translates into about two inches or five centimeters. • On a clean sheet of paper, write the name of each person—including yourself—who will take part in this experiment. You only need two people for this activity, but it's also great for a group. Leave five spaces below each name. Procedure • Hold the ruler vertically so that the zero end hangs down. • Ask your partner to stand next to you and place his or her hand below the ruler's zero line, ready to catch the ruler when it falls by pinching it between his or her thumb and index finger. Your partner's fingers should be just below the ruler, but as close as possible to the bottom edge without touching or overlapping. • Tell your partner that you will count from one to five and drop the ruler at some point during the count. Your partner will need to catch the ruler as quickly as he or she can, pinching the ruler between his or her fingers. • Count from one to five and drop the ruler at some point • Your partner should catch and pinch the ruler. How fast did your partner appear to act? Did your partner's fingers pinch near the zero line? • Write down the centimeter or inch line where your partner's fingers pinched the ruler. • Calculate how long it took your partner to respond using the chart provided. Was your partner as fast as you thought? • Repeat the drop four more times for your partner, and record the measurement each time. Does your partner's reaction time change? Are the five reaction times different? Vary when you drop the ruler: For example, you could drop on the count of five first, then drop on two. • Switch tasks and try catching when your partner drops the ruler, then compare your results with the others. Do most people have a similar reaction time? Are older people faster than younger people? Are girls faster than boys? • You can also try a few variations: What happens when you tell your partner when you will drop the ruler? Does reaction time improve with practice? • Extra : Ambidextrous, anyone? Repeat this activity and compare your results when you use your dominant hand—the hand you write with—and when you use your other hand. Is there any difference between hands? • Extra : Consider adding other distracting sounds and sights—such as turning on a TV set or flicking a flashlight on and off—during the activity. Do your responses slow with so many sensory signals? Observations and results Did you and your partner usually catch the ruler around 15 centimeters (six inches)? What took so long? On average, reaction time takes between 150 and 300 milliseconds. If that sounds like a long time, think about how much has to happen for you to react. When your eye sees the ruler falling, information travels from sensory cells called neurons from the eye to the brain's visual cortex, an area devoted to understanding what you see. Next, the motor cortex—the part of the brain that directs movement—has to send signals along your spinal cord and to your arm, hand and finger muscles, telling them to respond in the proper sequence to catch the ruler— quick! That's a lot happening in less than half a second—and a pretty amazing feat! More to explore: Experience versus Speed from Scientific American MIND Brain Brakes Car Faster Than Foot from Scientific American Reaction Time Test from the Human Benchmark How fast are your reactions? from the BBC
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Phys Ther Sci
- v.31(3); 2019 Mar
The effect of different visual stimuli on reaction times: a performance comparison of young and middle-aged people
Makoto otaki.
1) Faculty of Rehabilitation, Kobegakuin University, Japan
2) Division of Health Science, Graduate School of Medical Science, Kanazawa University: 518 Arise Ikawadani-cho, Nishi-ku, Kobe, Hyogo 651-2180, Japan
Katsuyuki Shibata,
3) Faculty of Health Science, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Japan
[Purpose] Here, we evaluated the reaction times of young and middle-aged people in different tasks. [Participants and Methods] The study included 23 young and 28 middle-aged volunteers. Their reaction times were measured in three tasks featuring different symbols (arrow and figure symbols) and spatial attributes (left, right, and ipsilateral choices). [Results] No significant inter-group differences in the reaction times were found for the simple reaction time task. In the choice reaction time and go/no-go reaction time tasks, the middle-aged participants demonstrated significantly slower reaction times. When the correct response was congruous with the direction of an arrow stimulus, the reaction times were shortened significantly among the middle-aged participants. In the go/no-go reaction time task, the reactions were delayed due to an inhibition of responses to upcoming stimuli. [Conclusion] The slower reaction time of the middle-aged participants in the choice reaction time task suggested that their responses were guided by the arrow stimulus to a greater extent compared to that of the younger participants. In the go/no-go reaction time task, the reaction times may have been slower in middle-aged participants because of a non-response possibility, which meant that participants had to first check the stimulus before deciding whether to respond.
INTRODUCTION
When driving a vehicle, we make split-second decisions based on instantaneous visual information. To perform appropriate actions in everyday life, we sometimes focus more on a particular stimulus, or we might delay our response to a stimulus to check whether the response is appropriate. According to Lewis et al., older people have diminished neuromotor function 1 ) . Reaction time has been used to measure age-related response quality 2 ) . There are three types of reaction time tasks: simple reaction time (simple RT), choice reaction time (choice RT), and go/no-go reaction time (go/no-go RT) 3 ) . MacDonald et al. found that older age groups exhibited longer reaction times and greater intra-individual variability in reaction time 4 ) . Many studies have compared the reaction times of young and old individuals 5 ) , but little is known about the reaction times of middle-aged people. Rehabilitative interventions cover a broad age range, so it is essential that such interventions are tailored to the response characteristics corresponding to the person’s age.
This study aimed to ascertain the characteristics of young and middle-aged people’s reaction times in different reaction time tasks from multiple perspectives. The ultimate goal was to provide findings that can guide rehabilitative interventions. To this end, we conducted two experiments. The first consisted of simple RT and choice RT tasks; the second consisted of a go/no-go RT task in which responses were inhibited.
PARTICIPANTS AND METHODS
We recruited 51 healthy volunteers, 23 of whom were young (9 males and 14 females; average age: 20.5 ± 0.6) and 28 of whom were middle-aged (16 men and 12 women; average age: 47.7 ± 8.0). We confirmed that each participant was right-handed using the Edinburgh Handedness Inventory 6 ) . Each of these individuals provided their informed consent to participate after receiving a briefing about the study’s purpose. The study was approved by Kobe Gakuin University’s ethics committee for human experimentation (No. HEB 17-15).
In the experiments, we used a personal computer, E-Prime 2.0, and Chronos (Psychology Software Tools, Inc.). When performing the tasks, the participants sat approximately 80 cm from the PC screen with their fingers poised on the response buttons ( Fig. 1 left). The participants were presented with a fixation point for 3,000 ms. After this, the target stimulus appeared and the participants had to execute a response ( Fig. 1 right). Each task consisted of a brief instruction, 10 practice rounds, and then 20 rounds in which we measured reaction times.

Experimental setting with an overhead view of response buttons (left), and visual stimulus samples (right).
The left figure shows the experimental setting, including the position of the desk, chair, and screen, with an overhead enlarged view of the response buttons. The right figure is a sample of the visual stimulus in the choice reaction task, indicates the direction of the passage of time. ←: Arrow symbol, ●: Figure symbol.
We only analyzed the reaction times of correct responses. We excluded reaction times of <100 ms deemed as “too early” and reaction times of >2,000 ms deemed as “too late.” The stimuli were randomized for each participant.
The statistical software we used was SPSS (version 24). We performed a multiple comparison (Bonferroni correction) on the three choice variables in the choice RT and go/no-go RT tasks (left/right, ipsilateral, contralateral). We used a paired t-test to compare reaction times between the two symbolic variables (arrow symbol, figure symbol) in the choice RT task. We used an unpaired t-test to compare the reaction times of the young and middle-aged participants. We set the statistical significance threshold at >5%. For the path analysis, we used SPSS Amos (version 24).
In the simple RT task, participants had to press a response button as quickly as possible when a stimulus appeared in the middle of the screen. In the choice RT task, each stimulus corresponded to one of three choice variables: left and right choice (LR choice), ipsilateral choice (IL choice), or contralateral choice (CL choice). For LR choice, the stimulus appeared in the middle of the screen and participants had to press either the left or right-hand button. For IL choice, participants had to press the left-hand button if the stimulus appeared on the left of the screen, and the right-hand button if it appeared on the right. For CL choice, participants had to press the right-hand button if the stimulus appeared on the left of the screen and vice-versa. The target stimuli consisted of two types of symbols: 1. an arrow (arrow symbol) that pointed in the direction of the button to press; and 2. a dot or cross (figure symbol) that did point in a direction. The arrow and figure symbols appeared at a ratio of 1:1, as did the two responses (left and right). The go/no-go RT task included the same three choice variables as the choice RT task—LR choice, IL choice, and CL choice. However, it also included a no-response condition (“no-go”). Two types of stimuli were used in this task: a black dot, which indicated that the response should be executed (“go”); and a white circle, which indicated that no response should be executed (“no-go”). The black dot and white circle appeared at a ratio of 1:1. The ipsilateral and contralateral choices appeared at a ratio of 3:2.
Table 1 shows the reaction times for the simple RT and choice RT tasks. In each task, around 1% of the responses were erroneous. None of the responses were too early or too late. To start with the young people, when the stimulus was an arrow, reaction times for the CL choice were significantly slower compared to that for the LR and IL choices (p<0.01). With a figure symbol, reaction times were slowest for the CL choice followed by the LR and IL choices. Reaction times were quicker with an arrow than with a figure symbol. Of the choice variables, the LR choice was associated with the quickest reaction times, and the CL choice with the slowest.
| Task ∙ symbol | Response | Young | Choice comparison | Symbolic comparison | Middle | Choice comparison | Symbolic comparison | Age comparison | |
|---|---|---|---|---|---|---|---|---|---|
| Simple | 232 ± 41 | 228 ± 41 | |||||||
| Choice | Arrow | LR choice | 314 ± 52 | 336 ± 53 | ** | ||||
| IL choice | 315 ± 64 | 344 ± 61 | ** | ||||||
| CL choice | 415 ± 92 | 439 ± 96 | ** | ||||||
| Figure | LR choice | 351 ± 69 | 372 ± 85 | ** | |||||
| IL choice | 309 ± 53 | 336 ± 60 | ** | ||||||
| CL choice | 391 ± 110 | 409 ± 84 | * | ||||||
| Go/no-go | LR choice | 333 ± 57 | 361 ± 64 | ** | |||||
| IL choice | 371 ± 74 | 415 ± 79 | ** | ||||||
| CL choice | 452 ± 122 | 498 ± 98 | ** | ||||||
n=51 (for each task).
All data are presented as the mean ± SD. Simple: Simple RT task; Choice: Choice RT task; Go/no-go: Go/no-go RT task; Arrow: Arrow symbol; Figure: Figure symbol; Response: Spatial attribute of button to press; Choice comparison: Comparison of left/right ipsilateral, and contralateral choice variables; Symbolic comparison: Comparison of the two symbol variables; Age comparison: Comparison of young and middle-aged participant responses; LR choice: Left and right choice; IL choice: Ipshilateral choice; CL choice: Contralateral choice. a: Left/right vs. Ipsilateral, b: Ipsilateral vs. Contralateral, c: Left/right vs. Contralateral, d: Left/right with arrow symbol vs. Left/right with figure symbol, e: Contralateral with arrow symbol vs. Contralateral with figure symbol.
††p<0.01, *p<0.05, **p<0.01.
As for the middle-aged participants, their reaction times were similar to the young participants overall. Although their reaction times in the simple RT task were not significantly different from that of the young participants, those in choice RT were significantly slower in the choice task in all conditions.
Table 1 also shows the reaction times for the go/no-go RT task. Among the young participants, reaction times were quickest for the LR choice (p<0.01) followed by the IL and CL choices. We observed a similar pattern in the middle-aged participants’ reaction times, but these reaction times were significantly slower than those of the young participants in all conditions.
To determine the difficulty level of each task variant, we compared the responses in all tasks on a Brinley plot 7 ) . The results revealed a linear relationship with an incline of 1.15, indicating that the reaction time of the middle-aged group increased as the difficulty level rose in each task. The most difficult task variants were go/no-go RT task with CL choice, arrow symbol with CL choice, and figure symbol with CL choice. We performed a covariance structure analysis (maximum likelihood estimation) to model the relationships of the task variants. The analysis of all measured variables yielded facilitating and inhibiting variables (χ 2 =33.6, p<0.01, RMSEA=0.023; Fig. 2 ). We extracted the variables based on the standardized coefficients. There was one facilitating variable: arrow symbol with LR choice. There were two inhibiting variables: 1. figure symbol with CL choice, and 2. go/no-go RT task.

Path analysis of facilitating and inhibiting factors.
The figures shown on the paths indicate standardized coefficients.
Simple RT: Simple reaction time; Choice RT: Choice reaction time; Go/no-go RT: Go/no-go reaction time; Arrow: Arrow symbol; Figure: Figure symbol; LR choice: Left and Right choice; IL choice: Ipsilateral choice; CL choice: Contralateral choice.
**p<0.01.
Age did not appear to influence reaction times in the simple RT task. In the choice RT and go/no-go RT tasks, however, reaction times were slower among middle-aged participants, who had more response choices associated with slower reaction times. Since response choices were more easily guided by an arrow symbol indicating a direction, more time was needed to determine selection of responses to left and right and whether to press the response button in tasks involving figure symbols.
Compared to figure symbols, the arrow symbol was associated with significantly quicker reaction times. According to affordance theory, instantaneous visual information elicits specific actions immediately without the mediation of complex cognitions or decisions 8 ) . Arrows are stimuli that contain information known by the perceiver such that attention is attracted in the direction of the arrow. As such, arrows can facilitate choice responses, which explains why the path analysis revealed the arrow symbol to be a facilitating factor for reaction times. Reaction times were significantly quicker in the IL choice than they were in the CL choice. DeJong et al. describes the response processes as follows; after the stimulus is presented, the stimulus is identified, the response is selected, and then the response is executed 9 ) . Responses are selected for stimuli during the response-selection step 10 ) . In the case of the IL choice, the response and stimulus are congruous (both are on the same side), so the response is selected automatically. In the CL choice, however, the response and stimulus are incongruous (they are on opposite sides), so the automatically selected response must first be inhibited, and then the response opposite to the automatic response must be selected. Therefore, in the CL choice, stimulus identification conflicts with stimulus response 11 ) . These procedural differences in response selection explain why the IL choice was associated with quicker reactions times while the CL choice were associated with slower reaction times. At higher difficulty levels, the go/no-go RT task was associated with slower reaction times than the choice RT task. In the choice RT task, every stimulus required a response. In the go/no-go RT task, however, only a “go” stimulus required a response (“no-go” required a non-response). According to Miller et al., a “go” decision activates a prepared response, but when “no-go” is a possibility, this prepared response must be inhibited until the person has checked whether it should be executed 12 ) . Accordingly, the go/no-go RT task increased the impetus to check the response against the stimulus. The presence of this checking process would explain why reaction times were slower in this task, as well as why the path analysis indicated go/no-go RT task as an inhibiting variable.
Reaction times were slower in general among both young and middle-aged participants; we noted an age effect. Given the tendency for an arrow symbol to elicit a response toward the direction it is facing, the presence of this symbol as the stimulus may have generated conflict between the spatial attribute of stimulus side and that of the response, resulting in delayed reaction times. The go/no-go RT task was more difficult because of the possibility of a no-go condition. The presence of the no-go condition may have delayed reaction times because the stimuli needed to be checked (as to whether it is “go” or “no-go”) before the response could be executed.
We only analyzed correct responses. Future studies should additionally analyze incorrect responses, including “go” responses to “no-go” stimuli. There is a trade-off between response time and response accuracy, and we did not control for this relationship in our study. Additionally, male may generally respond faster than females; such a gender bias shall be considered in the future studies which need to examine the effect in a more gender-balanced population.
Conflicts of interest
We have no conflicts of interest to declare.
Acknowledgments
We wish to thank all the students and faculty staff in the Faculty of Rehabilitation, Kobegakuin University who cooperated as participants.
Reaction Time Ruler
Activity length, activity type.
How fast can you react?
In this activity, the students participate in a simple ruler drop experiment and learn about the body’s response behind it.
When your friend drops the timer in the experiment, you see it start to move. A nerve signal travels from your eye to your brain then to your finger muscles. Your finger muscles move to catch the timer. The whole process takes between 150 and 220 milliseconds.
The neural pathway involved in a reaction time experiment involves a series of neural processes. This experiment does not test a simple reflex. Rather, this activity is designed to measure the response time to something that you see.
Catching a dropped ruler begins with the eye watching the ruler in anticipation of it falling. After the ruler is dropped, the eye sends a message to the visual cortex, which perceives that the ruler has fallen. The visual cortex sends a message to the motor cortex to initiate catching the ruler. The motor cortex sends a message to the spinal cord, which then sends a message to the muscle in the hand/fingers. The final process is the contraction of the muscles as the hand grasps the ruler. All of these processes involve individual neurons that transmit electrochemical messages to other neurons.
A person’s reaction time depends on a couple of things that can be improved and a couple that cannot.
Practice does make perfect because you can create a “muscle memory” that means you do not have to think so much to catch the ruler. You can take the time it takes to decide things out of the equation. Much of the time it takes you to react to the ruler dropping is the time it takes electrical signals to travel along your nerves. Moving at about 100 metres per second, a signal telling a finger to move has to travel from your brain down your spinal cord and into your arm. Signals for muscle control generally move faster than other ones. (Pain signals for example, move very slowly, often less than one metre per second). But these signals are “involuntary” which means that no matter how hard you try, you cannot control how quickly they occur.
The distance the reaction timer travels before you catch it has been converted to time using the equation d =1/2 a t² where a is the acceleration due to gravity.
This is a recommended pre-visit activity to Science World.
Describe how the nervous system responds to a stimulus.
Per Student Pair: copy of reaction timer template printed onto stiff card or attached to a ruler with tape
Key Questions
- How fast is your reaction time?
- What had to happen in your body for you to catch the ruler?
- How can reaction time be improved?
- Does your reaction time improve with practice?
- Why was the ruler caught in the middle (after a lag period) rather than at the end (instantaneously)? What causes this hesitation?
Preparation:
- Photocopy and cut out the reaction timer (double-check the size is accurate).
- Glue or tape it to a piece of stiff cardboard or ruler (unless printed onto card).
- Have students form partners for the activity.Each pair should decide who is number 1 and who is 2.
- Give each pair a ruler.

- Student number 1 will drop the ruler sometime within the next 5 seconds and student number 2 must try to catch the ruler as fast as they can after it is dropped.

- Swap positions so that student number 1 can test their reaction time.
Conversion Table (modified from Neuroscience for Kids):
| 2 in (~5 cm) | 0.10 sec (100 ms) |
| 4 in (~10 cm) | 0.14 sec (140 ms) |
| 6 in (~15 cm) | 0.17 sec (170 ms) |
| 8 in (~20 cm) | 0.20 sec (200 ms) |
| 10 in (~25.5 cm) | 0.23 sec (230 ms) |
| 12 in (~30.5 cm) | 0.25 sec (250 ms) |
| 17 in (~43 cm) | 0.30 sec (300 ms) |
| 24 in (~61 cm) | 0.35 sec (350 ms) |
| 31 in (~79 cm) | 0.40 sec (400 ms) |
| 39 in (~99 cm) | 0.45 sec (450 ms) |
| 48 in (~123 cm) | 0.50 sec (500 ms) |
| 69 in (~175 cm) | 0.60 sec (600 ms) |
- Explain that in order to catch the ruler a lot of messages have to be passed along different nerves:
- The eye sees the ruler drop.
- The eye sends a message to the visual cortex in the brain.
- The visual cortex sends a message to the motor cortex in the brain.
- The motor cortex sends a message to the spinal cord.
- The spinal cord sends a message to the hand/finger muscle.
- The finger muscle contracts to catch the ruler.
This happens almost instantaneously. How fast it actually happens is called the reaction time .
When comparing hands, students will usually find that their dominant hand is faster. Because the dominant hand is used more often every day, the neurons that carry messages between that hand and the brain are faster at transmitting electro-chemical signals. They are communicating along well-worn pathways. By running the same messages along the same pathway repeatedly, students can improve their motor skills. The phrase “practice makes perfect” is scientifically accurate.
- How did we know where the marks should go? Can you make a longer timer? Do you need to?
- Do students who play sports or musical instruments have faster reaction times?
- How does your reaction time change if you use your peripheral vision?
- Make the experiment more interesting by substituting candy bars (or another long snack) for the rulers. The students with the quickest reaction time get to eat the candy bar.
Other Resources
University of Washington | Faculty of Education | Neuroscience for Kids
About the sticker
Artist: Jeff Kulak
Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest.
Comet Crisp
T-Rex and Baby
Artist: Michelle Yong
Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape.
Buddy the T-Rex
Science Buddies
Artist: Ty Dale
From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way.
Western Dinosaur
Time-Travel T-Rex
Related Resources
Our sneaky senses, our senses tell us: what is out in the environmenthow much is out thereis there more or less of it…, colour and light, how are rainbows made what makes grass green and jeans blue how do sunglasses work in this series…, related school offerings.

Senses and Sensors

Visit BodyWorks Gallery

Eyeball Optics

Visit Puzzles and Illusions Gallery
We believe that now, more than ever, the world needs people who care about science. help us fund the future and next generation of problem solvers, wonder seekers, world changers and nerds..

1 Experimental Study of Human Reaction Time
Experimental study of human reaction time.
This lab is designed to align with AAOT science outcome #1: Gather, comprehend, and communicate scientific and technical information in order to explore ideas, models, and solutions and generate further questions.
- 12″ (30 cm) Ruler
- digital device with spreadsheet program
- digital device with internet access
- Apply a kinematic equation to predict the time required for on object to free-fall a certain distance.
- Apply a method to determine human reaction time and acquire experimental data on human reaction time.
- Analyze data to determine the average reaction time of a certain population.
- Analyze data to estimate the uncertainty in the average reaction time measured using this method.
- Analyze data to determine if reaction time depends on the number of tests a person attempts.
Experimental Methods
1) Have a friend or family member hold the ruler the top while you place your thumb and index finger about 3 cm apart on either side of the very bottom edge of the ruler, as if you were about to pinch the card between your fingers. Without giving any signal, the card holder will let go and you will close your fingers to catch the ruler. Whatever distance your fingers end up on when you catch the ruler, that is the distance the ruler fell while you reacted. This video shows what the experiment will look like, though our units and analysis methods will be different . You can then read the fall time for that distance from your spreadsheet. That was your reaction time. Record your first fall distance and corresponding reaction time value below. Also indicate any difficulties that you had in performing this experiment.
2) Repeat this experiment 10 times, recording your measured reaction time for each trial in a spreadsheet, which should look like the one below. Enter your drop distances in units of meters. Label the other columns as seen below but leave them blank for now.
| Trial | Fall Distance (m) | Reaction time (s) | Distance Uncertainty (m) | Upper Error (s) | Lower Error (s) | Time Uncertainty (s) |
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | ||||||
| 7 | ||||||
| 8 | ||||||
| 9 | ||||||
| 10 |
Modeling Methods
3) Find a kinematic equation that relates fall distance and time. Remembering the object was dropped from rest, rearrange the equation to isolate the time. Show your work below.
4) Apply the equation you found above within a spreadsheet formula to determine the ruler fall time for each drop distance in your dataset and fill in the column (don’t do all of the calculations by hand, we want to learn how to use the spreadsheet features). This fall time is how long it took you to see the ruler falling and close your fingers to catch it, which we will define as your reaction time in this study.
Analysis Methods
5) Graph the reaction time vs. trial number in a scatterplot. Give the graph a name and label the axes, including units.
6) Calculate the average, standard deviation, and standard error of the mean (SEM) of your 10 reaction time values. You may use the built-in functions of the spreadsheet to perform these calculations. The videos in the lab manual introduction demonstrate these calculations.
7) The standard deviation tells us about variation in the data (how close together the value are). In other words, the standard deviation represents the lack of consistency in your reaction time from one trial to the next. Due to the lack of consistency, we would not be very certain that a small number of reaction time measurements would be representative of your average reaction time. However, the uncertainty in the measurement of an average value can be reduced by averaging many individual measurements. That uncertainty is often estimated by the SEM (based on the assumption that the variation in the values is random). Using the SEM as an estimate of the uncertainty in your average reaction time, report your average reaction time with uncertainty in the standard format: average + uncertainty in the average.
8) Calculate a percent uncertainty in the average. Report your average reaction time with % uncertainty in the standard format: average + percent uncertainty in the average (%).
9) Apply a trendline to the plot of the data and display the trendline equation and R 2 value on the graph, and record each here:
10) Do the data suggest that there is a trend (correlation) in the reaction time vs. trial data? Explain in terms of the error bars, the trendline equation, and the R 2 value.
Conclusions
11) If a significant amount of the variation in the data is actually caused by a real trend in the data (such as getting faster or slower with more trials) then you did not actually attempt to measure the same thing 10 times (reaction time), you measured 10 different things one time each (reaction time after different amounts of practice). In that case the variation is caused by the trend, not by measurement error or random inconsistency in your reaction time so we cannot trust that the SEM is representative of the uncertainty in the average value we found. Based on your previous answer, do you feel that the SEM is a good estimate of the uncertainty in your average reaction time?
12) Are you confident that the average reaction time value you measured is representative of your actual typical reaction time? Explain your reasoning, which should incorporate your answers above and the SEM value.
Further Questions
13) Verification/replication of scientific results is an important part of the scientific process. Use this online reaction time tester to quickly make another 10 reaction time measurements. Find the average, standard deviation, and SEM of those 10 results and record below.
14) Contrast the online tester results with those of your fall-time experiment. Were the average reaction times measured by each method in agreement? Explain. (Do the average + SEM of each result overlap?)
15) Find a peer-reviewed research article on human reaction time and compare the result of that study to your result and the online reaction tester result. Does your result seem reasonable in comparison? Explain. Do any of the average results agree within the combined uncertainty in your measurement and theirs? (Do the average + uncertainty of the result overlap?) Explain.
16) Do your results suggest that the fall-time method is a reasonable way to test reaction time? Explain by referencing specific results of this lab and comparisons with other methods.
Body Physics Remote Lab Manual Copyright © by Lawrence Davis. All Rights Reserved.
Share This Book
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School
How to test your reaction time
August 31, 2022 By Emma Vanstone 1 Comment
Do you think you have fast reactions? Have you ever measured your reaction time? Did you know you can test your reaction time using just a ruler?
Reaction time is the time it takes for a person to respond to a stimulus. For example, if you touch something very cold, there is a slight delay between touching it and moving your hand away. This is because it takes time for the information to travel from your hand to your brain, where it is processed. Many sports and activities require fast reactions!
What’s the difference between a reflex and a reaction
Reactions are different to reflexes which are involuntary. Reflexes are faster than reactions.
How to test reaction time with a ruler
You can test reaction time s using just a ruler.
Simple ruler drop reaction time test
What you need.
Pen and Paper
Hold the top of the ruler with your arm stretched out. Your fingers should be on the highest measurement.
Ask a friend to put their thumb and index finger slightly open at the bottom of the ruler, with the ruler between their fingers. They need to grab the ruler as soon as it drops.
Drop the ruler and record the measurement on the ruler where the other person’s fingers are.
Repeat for all participants. Let each person have three attempts and record the average value.
The person with the fastest reaction time is the one who catches the ruler at the lowest measurement, as the sooner the ruler is caught, the less time it has to fall.

How does this work?
Our eyes see that the ruler has been dropped and send a signal to the brain, which sends a signal to the muscles in the arm and hand to tell them to catch the ruler. Our body is very clever, and these signals travel very, very quickly.
Information from the eyes is sent to the brain and then to the hand via neurons. The brain processes the information and decides what to do next. The human brain contains around 100 billion neurons!
Your reaction time depends on the time taken for the signals to travel between your eye, brain and hand.
Reaction Time Challenges
Design a table to record the results.
Investigate to discover whether reaction time can be improved with practice. Does muscle memory help speed up your reaction time?

More Reaction Time Tests
Repeat the investigation using your non-dominant hand to investigate whether this makes your reaction time slower.
Design an investigation where you work out the average reaction time for different age groups.
Tie a piece of string to a toy car and let it run down a ramp. Measure how far the car travels before a person can stop it.
Can you think of any more ways to test reaction time ? What would you consider a slow reaction time?
Print the reaction time template below to see how fast your reaction times are!
Learn more about the brain with our play dough brain model .
If you like this activity, you might also like our collection of sporty science experiments for kids .
Quick Summary
Reaction time is the time it takes you to react to a stimulus .
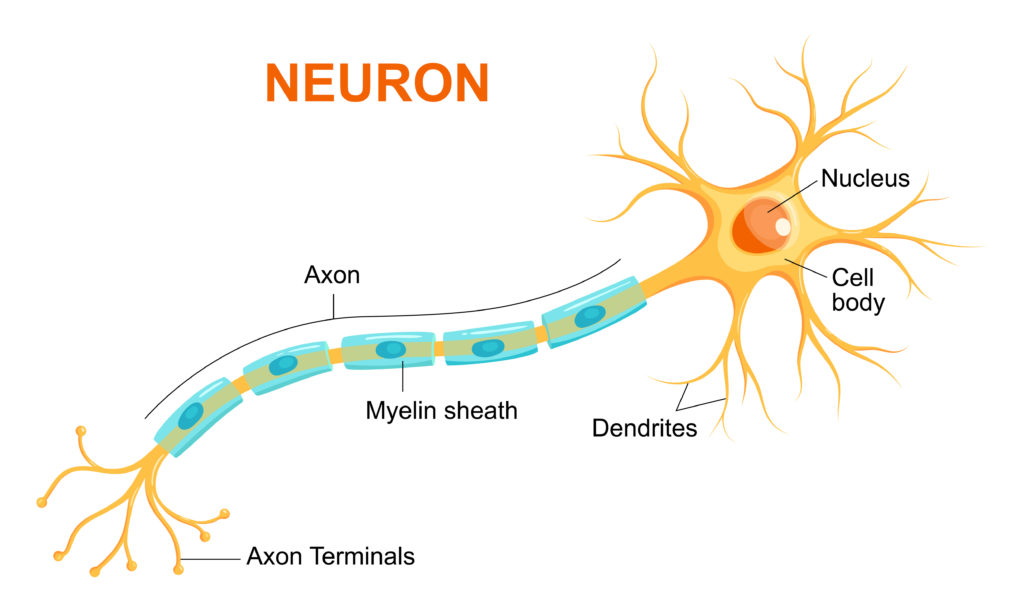
Information is sent around the body via nerve cells called neurones . These form the peripheral nervous system . The central nervous system consists of the brain and spinal cord.
Science Concepts
- Reaction time
- Nervous system

Last Updated on May 23, 2024 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
November 05, 2019 at 7:41 pm
I was going to do a grade 8 science fair. and I decided to do reaction times. Thank you for giving me a way to test reaction times!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Sorry, your browser must support frames to view this material. Try Firefox , Safari, or Netscape.

# Precise Timing
# background & context.
Reaction time in psychology research is used to quantify cognitive processes and behaviors. A clear-cut definition of reaction time has to do with the amount of time passed between an appeared stimulus and the response.
There are two components to measuring reaction time, the stimulus’ time of onset and when the participant’s response occurred, illustrated by Fig.1.

Fig. 1: The two main components of measuring reaction time.
For reaction time to be measured accurately, the exact time of the stimulus onset (Point A) must be known, as well as when the participant’s response (Point B) happened as reaction time is the difference between these two points. From the two points, it is easy to determine when a participant’s response occurred, but it is challenging to know exactly when the exact stimulus onset occurred (Point A).
Why is it challenging to determine when Point A occurs? There are three main reasons that influence when a stimulus appears:
Screen refresh rate: The rate of monitor refreshing occurs at 60Hz so if something is scheduled to occur, it can occur only when the monitor is refreshed. While this is on a millisecond scale, it’s an important factor to quantify (which we discuss later how it is measured with the request animation frame) as it directly impacts the experimental sequence.
Nature of programming: All experiments are based on coding and for code to be executed, it must be processed as nothing is instantaneous, this usually takes 1-2 refresh cycles.
Device capacity: Though this is not common, if the participant’s device capacity is really slow, the stimulus presentation can lag as all of the system delays (like a computer freeze). We discuss later on how we check for this issue (the JavaScript Event Loop).
In summary, reaction time is affected by many factors upon which technological processes are built in order to accurately determine the time between stimulus onset and the participant’s response.
# Publication in Behavior Research Methods :

# Our Process: Labvanced’s pipeline for precise timing

Fig. 2: The general pipeline for precision timing and capturing accurate reaction times in Labvanced.
To provide precise timing and reaction times, our software follows these steps (Fig. 2) :
Preloading (caching): Ensuring all experimental stimuli are loaded a priori to the experiment beginning and locally available so loading does not happen in the midst of experimental progress. So, if a participant wants to take part in a study, all the stimuli (images, audio,and video) are already fetched and loaded locally on their computer from our server.
Pre-rendering: When the experiment begins, the content is recursively created so that the next frame and trial is loaded in the background and ready to go as soon as the participant is ready to move on. This is driven by a pre-rendering mechanism.
Participant-Specific Measurements: Since online studies begin in the browser, each participant has finite computer resources (GPU, CPU) which must be kept under consideration as they affect performance. We capture any potential delay and provide it as a correctional variable to the researcher which can also be used as an exclusion criteria.
# Saving Participant’s Responses

However, if the provisions are available, our software is set up so that data recording and responses are saved automatically after each trial. This is important because:
- A local browser cannot hold or cache an infinite amount of memory. By backing up frequently, memory is freed and the system does not risk lagging.
- If a participant stops or drops out, there is at least some data saved for the trials that they did complete and provide responses to prior to terminating their participation.
# About the Timestamp

# About System Architecture and Reaction Time Data Flow
While the pipeline described above captures the basic steps of the reaction time process, below is a more detailed explanation of everything that is going on in Labvanced to make the reaction time measurement accurate and precise.
# Preloading (Caching)

Fig.3: The main steps of the preloading/caching mechanism in Labvanced.
Preloading or caching occurs before the experiment even begins. Labvanced is set up so that all of the study’s experimental stimuli are downloaded before the study starts. This includes all of the elements, such as images and videos. They are all fetched from the Labvanced servers and downloaded locally to the participant’s device so that no downloading has to occur during the experiment itself (Fig. 3).
# Pre-Rendering Mechanism

Fig. 4: The main steps of the pre-rendering mechanism in Labvanced.
We have a pre-rendering mechanism in place to build the structure of the experimental tasks, trials, and frames in advance. For example, if you are in Trial #1 of a task, we pre-render all frames in the current and upcoming trial so that loading does not happen during the experiment, including the instruction, text, audio objects, fixation cross, etc. By building the trials and frames in advance, it prevents the browser from slowing down or being overwhelmed (Fig. 4).
# Participant-Specific Measurements
Because of the innate variability between devices and computers, performance is affected by the definition. Simply by running an experiment on a local system which are inherently limited with resources (ie. speed and memory are not infinite but constrained by their tech specifications), stimuli may not get shown as expected (there may be a delay of a few milliseconds, for example.
To capture these device- and participant-specific fluctuations, we have the following mechanisms in place:
- The request animation frame
- The JavaScript Event Loop

# Request Animation Frame

Fig. 5: Demonstration of the request animation frame mechanism in Labvanced.
Every 60ms the monitor is independently updating and refreshing, this is a constant for all computers and screens. To determine whether there is a delay in presentation of the stimulus (on the millisecond scale), the request animation frame is used for all instances where a timed stimulus is occurring.
Let’s say you execute code to show stimuli at 2000ms, when you execute it nothing happens, the stimuli will be automatically presented at the next refresh rate, 60 milliseconds (Hz) later, at the 240ms mark. You can measure this tiny lag and account for it post-hoc. Because we use the request animation frame, you can know exactly when a command was executed (when it really happened/appeared on the monitor) and adjust accordingly (Fig. 5).
# JavaScript Event Loop

Another example of participant-specific measurements has to do with determining the speed of their device.
If your computer is slow, it may be because there are active system processes running that use available CPU. Thus, the browser is working the limited resources that are available and as a result, everything gets slower.
To determine whether this is happening on the participant level, we use the ** JavaScript Event Loop using CallBack Functions** which runs automatically (by default) in the background to measure the amount of time it takes for the function to call back on itself. If it doesn't return within 5 ms, it means the participant’s browser/computer is slow which could affect the integrity of experimental results measuring reaction time (Fig. 6) . We report the mean value in milliseconds that it takes for the CallBack Function to return for the participant.
For the thousands of studies that have been completed by participants in Labvaned, we have found that over 95% of participants have a reported value that falls below 3ms, sometimes below even 1ms. But in some cases, there are results that average 200-300ms which could indicate to the researcher to consider excluding that particular user’s data from the final data set analysis.
# Key Features of Labvanced’s Reaction Time and Precision Timing Capabilities:
Our top features for measuring participants’ responses include (Fig. 7):
- Temporal accuracy of stimulus presentations
- Spatial accuracy of stimulus presentations

Fig. 7: The key features of Labvanced’s precise timing / reaction time solution.
# Advantages of Labvanced’s Precision Timing
Because of these steps and mechanisms, Labvanced offers an accurate and precise solution to measuring reaction time during online experiments. We highlight the following advantages of our platform:
- Controlled timing of stimuli: Researchers have knowledge of the exact time that stimuli are presented on screen, allowing for adjustment and accurate measurements.
- Strong computational and programming mechanisms: To assure the researcher the most accurate data is being reported, we use strong computational and programming mechanisms in order to accurately quantify the onset of stimuli on the participant’s screen.
- Tried and tested: We have worked with researchers from all over the world to fine-tune our platform and as a result our features have been tried and tested by countless of research and academic institutions using our online reaction timing measuring as a basis for their studies and published works.
# Sample Data & Metrics for Reaction Time

Fig. 8: Data report from a participant’s session performing the Stroop Task using Labvanced; 3rd column from the right demonstrates recorded reaction times.
Things You Can do with Labvanced’s Precision Timing:
- Cognitive decline
- Performance measures
- Feature recognition

open in new window
# LV Library Studies:
There are many studies that measure how long it takes for a response to a stimulus to occur, here are a few examples of tasks that have reaction time measurement at their core:
Or search by topic
Number and algebra.
- Place value and the number system
- Fractions, decimals, percentages, ratio and proportion
- Calculations and numerical methods
- Algebraic expressions, equations and formulae
- Coordinates, functions and graphs
- Patterns, sequences and structure
- Properties of numbers
Geometry and measure
- 3D geometry, shape and space
- Transformations and constructions
- Vectors and matrices
- Measuring and calculating with units
- Pythagoras and trigonometry
- Angles, polygons, and geometrical proof
Probability and statistics
- Handling, processing and representing data
- Probability (spec_group)
Working mathematically
- Thinking mathematically
- Mathematical mindsets
Advanced mathematics
- Decision mathematics and combinatorics
- Advanced probability and statistics
For younger learners
- Early years foundation stage
Reaction timer
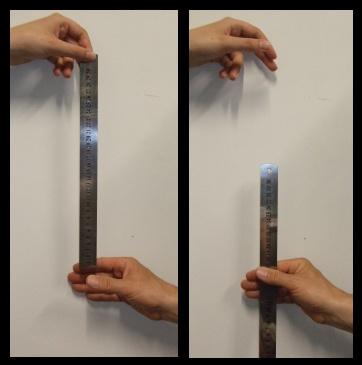
Here are some questions you might like to consider:
- I think I respond more quickly with my right hand than with my left - are you the same?
- Do your reactions vary depending on the time of day or the sort of thing you are being asked to react to?
- Do your reactions improve with training/practice?
- Do boys react more quickly than girls?
- Do young people react more quickly than older people?
- What else do you think affects people's reaction times?
- Are both experiments testing the same ability? If you perform both experiments with a group of people and rank them in order, will the rankings be the same for both experiments?
- Can you think of other experiments you could do to test your reactions?
You may also wish to explore how accurately you can estimate time .
We received a number of observations and conjectures.
Octavia from Fowlmere suggested the following
- Right handers are better at reacting with their right hand and left handers vice versa
- At night you are more tired so your reactions are slower
- Males tend to be quicker than females because they play more computer and playstation games
- The older you are the more time it takes for you to react because your body doesn't work as fast
Meghan from AHS suggested that if you change the properties of the star, it is harder to click it quickly. She also added that males are actually no quicker than females. We already have conflicting conjectures, and this is where providing real data in support of your argument is important.
Maria and Katie from St Mary's conducted an experiment, in which they found that the average reaction time for their left hand was 0.2s, while for their right hand it was 0.15s. Rosie, Natalie and Gabby, also from St Mary's provided similar data which supports the argument that we react quicker with our better hand.
Michael from Lancaster Grammar experimented with a moving star. He made the following acute remark: "If you are right handed have the mouse at the right side of the screen, so when a star does come it is easier to get to the star because your right hand can move faster and more easily to the star if it is at the left."
This raises an important issue - that factors other than reaction time (such as strategy) can affect our results. In conducting a fair experiment it is essential to make sure that these other factors are controlled.
In response to our demand for experimental data, a number of students from Chalkstone Middle School sent in their findings. Sithabile and Shannon sent in some data and concluded that we react fastest with our best hand. Kelly, however, claimed that people always do better with their left hand. Keeley claimed that boys have better reactions than girls while under stress, but otherwise girls are quicker!
The data were well organised and clearly presened, but in many cases we were concerned that there were not enough data to truly back up your claims. A number of you based your conclusions on testing each individual in your sample just once. Aaron and Eshter made an effort to get more accurate results by repeating individual experiments three times.
To learn more about collecting data and making conjectures, we suggest reading Understanding Hypotheses .
Why do this problem?
The skills of making and testing hypotheses and analysing data are important both in mathematics and in scientific enquiry. This problem is an ideal starting point for developing these skills.
Learners need to make decisions about the information that is required to answer the questions posed, analyse the data that is collected, and decide whether the analysis supports the hypothesis.
Possible approach
To introduce the second experiment, ask a volunteer to come out to the front of the class and demonstrate dropping a ruler to test the speed of their reactions.
Once learners have seen both experiments, give them some time to discuss in pairs some hypotheses they could test, and then share these ideas with the whole class. There are some suggested lines of enquiry in the problem which could be shared with learners if they struggle to come up with good ideas of their own.
- whether they think it is true or false
- how they could use the experiment(s) to test their views
- what data (and how much data) they would need to collect
Give them time to collect, analyse and interpret their data and then prepare a poster for presenting their findings to the class. The task may take more than one lesson, so data collection could be done as a homework task. One way of presenting their findings to the class is for learners to display their posters around the room and then take time to look at everyone else's work, perhaps annotating each other's work with post-it notes. Then the class could discuss which methods of collection, analysis and representation were most appropriate and effective in testing their hypotheses. Another similar activity where students can make hypotheses and test them is Estimating Time .
Key questions
How many times do you think it would be useful to carry out the experiment(s)? How will you represent and analyse your data to test your hypothesis? Can you justify that your experiment is a valid way of testing your hypothesis? Are your results reliable - could someone else replicate your results with their own experiment?
Possible support
Encourage learners to work in pairs or small groups and to support each other in constructing clear hypotheses which are straightforward to test. Each group could present their plan to the rest of the class before they start any data gathering, and the class could give feedback on what is good and what might need improving. This could be done using post-it notes as suggested above.
Possible extension
All of the hypotheses suggested in the problem could lend themselves to fairly detailed statistical analysis - there is the opportunity for learners to explore the idea of distributions, averages and measures of spread in order to compare data gathered from each of the two experiments and any experiments they devise for themselves.
A Stage 4 follow-up problem that investigates how to turn the results from the second experiment into reaction times can be found at How Do You React?
Reaction Time Test
Sequence Memory Test

Aim Trainer

Number Memory Test

Visual Memory Test

Matching Game
Display headings.
This test analyzes your reflexes and measures how fast you can react to the on-screen prompts. It precisely calculates how fast you click and displays the result in milliseconds. The average score of this reaction time test is 273 ms. A lower number means your reaction to the on-screen prompt took less time to click. A higher score means you were slower to react and click.
So, if you score lower than 273 ms, you are already in a good place. However, if you scored a higher number, you will need to practice more and hone in on your reflexes. Also, the score is a bit exaggerated by the latency of your computer. When you click on your mouse, the signal from the mouse travels through the system and is then shown on the display.
This process can take about 10–50ms, which is also added to the score. Without the computer latency, your reaction time score could be even better. Using a high refresh rate monitor with a faster computer will result in a better score. Also, avoid doing this test if your computer is connected to a TV. That’s because TVs can have over 100 ms of latency, pushing the score into a worse category.
| Test Number | Reaction Time |
- Summer Sports
Australian B-Girl Raygun 'devastated' by online hate after Olympic performance
Multiple theories have sparked vitriol aimed at rachael gunn after her olympic breaking debut.

Raygun ‘devastated’ by online hate after Olympics performance
Social sharing.
Rachael Gunn, also known as B-Girl Raygun, spoke out Thursday after several whirlwind days of memes, accusations and conspiracy theories surrounding her performance at the 2024 Paris Olympics.
In a video post on Instagram , Gunn thanked her supporters but said the hate she has received online "has, frankly, been pretty devastating."
"I went out there and I had fun. I did take it very seriously. I worked my butt off preparing for the Olympics, and I gave my all, truly," she said.
Gunn's Olympic performance went viral for all the wrong reasons.
Memes mocking the Australian dancer's breaking moves at the Games have flooded the internet since she lost all three of her round-robin battles by a combined score of 54-0, in a performance remembered for her "kangaroo hop" and other moves that perplexed audiences.
But the online discourse surrounding Gunn, also known as B-Girl Raygun, has shifted into something more malicious.
Social media users, confused by how Gunn made her way to the world stage, have made accusations that she rigged the competition to qualify for the Olympics, that she intentionally bombed her performance and that she's the reason breaking won't be returning to the 2028 Olympic Games in Los Angeles — even though that decision was made before the 2024 Games started.

How does breaking work at the Olympics?
How did gunn qualify for the olympics .
Gunn's critics have falsely claimed that she and Samuel Free, her coach and husband, founded the organization that ran the Australian competition where Gunn qualified for the Olympics.
This theory has gained plenty of traction online. A change.org petition demanding a public apology for alleged "unethical" behaviour by Gunn and Australian Olympic boss Anna Meares had more than 57,000 signatures before it was taken down Thursday.
"Rachel [sic] Gunn, who set up her own governing body for breakdancing, has manipulated the selection process to her own advantage," the petition claimed.
The petition called for a "full investigation" into the selection process, an audit of Gunn's "business dealings" and a public apology from Gunn and Meares for "misleading the Australian public and attempting to gaslight the public and undermining the efforts of genuine athletes."
The Australian Olympic Committee (AOC) wrote to change.org demanding it take the petition down. "It amounts to bullying and harassment and is defamatory," CEO Matt Carroll said in a statement.
- Australian Olympic Committee hits out at criticism of controversial breaker Rachael 'Raygun' Gunn
- From Phil Wizard to Raygun, breaking is bigger than ever. Is that a good thing?
The AOC also clarified that Gunn and Free hold no positions with AusBreaking or DanceSport Australia in any capacity, and that Meares was not involved in the qualifying event or the nomination of athletes.
Following her performance Saturday, Gunn told media that she tried to be creative, because she couldn't compete athletically with her younger rivals.
"All my moves are original," she said. "Creativity is really important to me. I go out there and I show my artistry. Sometimes it speaks to the judges, and sometimes it doesn't. I do my thing, and it represents art. That is what it is about."
The allegations prompted AusBreaking, the organization that ran Australia's qualifying competition, to release a statement Tuesday saying the selection process for Australia's Olympic breaking team was open to all interested participants and adhered to World DanceSport Federation (WDSF) regulations.
A panel of nine international adjudicators, a head judge and a chairperson oversaw Australia's qualifying competition, using the same judging system as the Paris Games. Free was not one of the judges for the event. In fact, none of the judges were even Australian .
The WDSF Oceania Championships drew 37 male and 15 female entrants, from which Gunn and male competitor Jeff Dunne, a.k.a. J-Attack, emerged victorious.
- Breaking makes Olympic debut as Japan's Ami takes women's gold
- Canada's Phil (Wizard) Kim captures Olympic gold medal in men's breaking
"Their selection was based solely on their performance in their battles on that day," the statement read.
"We condemn the global online harassment and bullying of Raygun. The pressure to perform on the Olympic stage is immense, especially against the opponents in her particular group. We stand in solidarity with Raygun."
AusBreaking — originally called the Australian Breaking Association — was founded by breaking champion Lowe Napalan in 2019. Gunn and Free are not listed as executive members or committee members of the organization. A spokesperson declined to answer questions, saying AusBreaking will be open to interviews "once key conspiracies have been addressed."
Shocking story. Also happens not to be true. Neither Gunn nor her husband Samuel Free is a founder of AusBreak, nor are they on its board.<br><br>Not sure quite how people find these claims that don't stand up to a quick search. <a href="https://t.co/2x0ZD3SdUN">https://t.co/2x0ZD3SdUN</a> — @charlesarthur
However, several Australian breakers told The Guardian that a number of issues kept many of the country's best B-girls from taking part in the Olympic qualifying competition, leading to a contest that was poorly attended and missing top talent.
The event was held shortly after it was announced, the B-girls said, and participants had to register with three different bodies to sign up. The competition also required registrants to have a valid passport, which many did not.

Will we ever see breaking at the Olympics again?
Others have since spoken out to defend her from the online criticism.
Martin Gilian, the head judge of the Olympics breaking competition, said Sunday she did her best and was simply not as good as her competitors.
"Breaking is all about originality and bringing something new to the table and representing your country or region," Gilian said at a press conference. "This is exactly what Raygun was doing. She got inspired by her surroundings, which in this case, for example, was a kangaroo."

Meares, the Australian Olympic boss, has also spoken out against the online comments.
"I love Rachael, and I think that what has occurred on social media with trolls and keyboard warriors, and taking those comments and giving them air time, has been really disappointing," Meares told a news conference Saturday.
Invented stories can be damaging
Jeffrey Dvorkin, a senior fellow University of Toronto's Massey College and former journalist, says false narratives spread quickly online because they are often more interesting than the original, or true, story.
"I think that what we're seeing now is the story is so amazingly trivial in the long run that people start to invent side stories around it to make it more interesting, but not necessarily more credible," Dvorkin told CBC.
- Olympic breaking had its moment. But will we ever see it at the Games again?
- Breakers look to capture — and keep — the world's attention at Olympics
He says people look for elements in online content that confirm their own biases, so they may be quick to share something that feels correct in their mind, without bothering to check whether it's credible. People do this in part to combat the alienation created by the internet and build their identities, he says, "at a time when we are being fragmented into a million different pieces and places."
He says reposting something on the internet, true or false, gives social media users an endorphin rush . "So it makes people feel better about themselves, even when they are spreading misinformation."
Those momentary positive feelings for social media users may come at the expense of their object of ridicule.

Olympic breaking highs and lows in Paris
Sergey Nifontov, general secretary of the WDSF, expressed concerns about Gunn's mental health, and said the federation has contacted her and Australian Olympic team officials to offer support.
"We offered [the] support of our safe-guarding officer. We are aware about what has happened, especially on social media, and definitely we should put the safety of the athlete — in this case mental safety — in first place," he said. "She has us as a federation supporting her."
Dvorkin says this kind of internet mob mentality can be "very damaging and very, very destructive."
"People are are made to suffer for the misinterpretation inflicted on them by others," he said.
- Phil (Wizard) Kim's breaking gold helped introduce sport to the world, mentor says
- Breakdancing's Olympic debut inspires new generation of young breakers
Gunn referred viewers of her Instagram video to the statements from AusBreaking, the AOC and the WDSF "in regards to the allegations and misinformation floating around."
"I'd really like to ask the press to please stop harassing my family, my friends, the Australian breaking community and the broader street dance community," she added. "Everyone has been through a lot as a result of this."
Breaking will not return at the 2028 Los Angeles Olympic Games, but that has nothing to do with Raygun's performance.
Each host city has an opportunity to bring in several new sports, and L.A. had already selected theirs before the Paris Games began. The 2028 Olympics will add flag football, lacrosse, cricket, squash and baseball-softball.
ABOUT THE AUTHOR

Digital Writer
Kevin Maimann is a senior writer for CBC News based in Edmonton. He has covered a wide range of topics for publications including VICE, the Toronto Star, Xtra Magazine and the Edmonton Journal. You can reach Kevin by email at [email protected].
With files from The Associated Press and Reuters
Related Stories
More From Forbes
Raygun, the australian breakdancer in the olympics: explained.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Raygun competes during the Breaking B-Girls Round Robin Group B battle between Logistx and Raygun on ... [+] Day 14 of the Olympic Games Paris 2024 at La Concorde on August 9, 2024 in Paris, France. (Photo by Harry Langer/DeFodi Images via Getty Images)
Aussie breaker Rachael Gunn , known as B-girl Raygun, took the internet by storm after her brief but memorable time on stage at the Paris 2024 Olympics. In head-to-head battles against b-girls from the USA, France, and Lithuania, she pulled out some unique moves like kangaroo hopping and swimming on the ground.
Some netizens were less than impressed, posting messages like “There’s 27.7 million Australians in the world and that’s who they send to the Olympics for this inaugural event??? C’mon now!” Others expressed doubt about breaking’s inclusion in the Olympics.
While the memes are admittedly funny, there are two misconceptions about breaking and B-girl Raygun that are important to clear up.
Misconception No.1: “This Is What Olympic Breaking Looks Like”
The beauty of breaking lies in its freedom of self-expression. Among all the Olympic sports, breaking has perhaps the most opportunity for creativity. At the same time, there is a sky-high ceiling for physical and technical ability. Look at the performances of the medal winners Ami, Nicka , and 671—you’ll see more gravity-defying, dynamic sequences than you can throw a shoe at.
Best High-Yield Savings Accounts Of 2024
Best 5% interest savings accounts of 2024.
On the other hand, there’s Raygun’s approach. She herself told reporters , “I was never going to beat these girls on what they do best, the dynamic and the power moves, so I wanted to move differently, be artistic and creative because how many chances do you get that in a lifetime to do that on an international stage.”
She came into the competition with a goal of making her own mark, and in a sense, she accomplished that.
Misconception No.2: “Raygun Should Not Have Qualified For The Olympics”
In the IOC’s own words , “the Olympic Games are the world’s most powerful symbol of unity in all our diversity.” The Olympics include diverse participants from almost every part of the world, which naturally leads to situations where certain athletes are outclassed by others. No country excels in every single sport, and in breaking’s case, Australia is simply not as competitive.
That doesn’t take anything away from the time and effort that it took Raygun to get to the Olympics. She secured a spot by winning the 2023 Oceania Breaking Championship , and she represented Australia at the 2021 and 2022 World Championships. Her style wasn’t enough to pass the group stage in Paris, but she is undoubtedly a qualified representative for her region. The 36-year-old has been breaking since her 20’s and is known as Dr. Gunn when she’s at her day job: lecturing on dance and gender politics at Macquarie University.
At the end of the day, it’s all about positivity:
“It was amazing. Such an amazing experience,” Gunn told Yahoo Sports after the event. “What a stage, what an arena, what a crowd. Music was great. Like, oh, so, so grateful for the opportunity.”
Breaking will take the Paris Olympics stage again on August 10 with the B-Boy (Men’s) event.

- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 August 2024
Elucidation of hemilabile-coordination-induced tunable regioselectivity in single-site Rh-catalyzed heterogeneous hydroformylation
- Benhan Fan ORCID: orcid.org/0009-0005-5606-3873 1 na1 ,
- Miao Jiang 1 na1 ,
- Guoqing Wang 1 ,
- Yang Zhao 1 ,
- Bingbao Mei 2 ,
- Jingfeng Han 3 ,
- Cunyao Li 1 ,
- Guangjin Hou ORCID: orcid.org/0000-0001-8216-863X 4 ,
- Li Yan ORCID: orcid.org/0000-0002-6298-467X 1 &
- Yunjie Ding ORCID: orcid.org/0000-0001-8894-9648 1 , 4
Nature Communications volume 15 , Article number: 6967 ( 2024 ) Cite this article
662 Accesses
3 Altmetric
Metrics details
- Catalytic mechanisms
- Heterogeneous catalysis
- Solid-state NMR
Revealing key factors that modulate the regioselectivity in heterogeneous hydroformylation requires identifying and monitoring the dynamic evolution of the truly active center under real reaction conditions. However, unambiguous in situ characterizations are still lacking. Herein, we elaborately construct a series of Rh-POPs catalysts for propylene hydroformylation which exhibited tunable regioselectivity. Multi-technique approaches reveal the unique microenvironment of the diverse HRh(CO)(PPh 3 -frame) 2 sites with distinct P-Rh-P bite angles ranging from 90° to 120° and 158° to 168°, respectively. In situ time-resolved XAFS, FT-IR, and quasi-in situ Solid-state NMR experiments combined with DFT calculations explain the dynamic evolution of the electronic and coordinate state of the distinct active sites induced by hemilabile PPh 3 -frame ligands and further disclose the regulatory mechanism of regioselectivity. These state-of-the-art techniques and multiscale analysis advance the understanding of how hemilabile coordination influences regioselectivity and will provide a new thought to modulate the regioselectivity in future industrial processes.
Similar content being viewed by others
Promoting active site renewal in heterogeneous olefin metathesis catalysts
Rh single atoms on TiO2 dynamically respond to reaction conditions by adapting their site
Isolated Rh atoms in dehydrogenation catalysis
Introduction.
Hydroformylation, a representative homogeneous atomic economic reaction, is a crucial industrial process of forming aldehydes from alkenes and syngas. Moreover, aldehydes can be used in the preparation of alcohols, amines, carboxylic acids, and other high-value-added fine chemicals 1 , 2 . Up to now, the annual production of aldehydes and further hydrogenated alcohols by hydroformylation has exceeded 25 million tons and the industrial processes make use of homogeneous rhodium–phosphine complex catalyst with the issues of product separation, discharge of wastes containing phosphorus and loss of noble metals 3 , 4 . Heterogeneous single-metal-site catalysts (HSMSCs) have well-defined active centers and customized local coordination environments, which could improve the stability and utilization efficiency of precious metal 5 , 6 , 7 , 8 . Meanwhile, the evolution of the whole reaction process can be clearly characterized with the superiority of the clearly active center and this new perspective provides an opportunity to bridge the gap between homogeneous and heterogeneous hydroformylation. Recently, some instructive studies have been created for designing efficient hydroformylation catalysts by atomically dispersed metal atoms anchored on diversiform solid supports, such as zeolites 9 , 10 , 11 , 12 , 13 , inorganic oxides 14 , 15 , 16 , 17 , carbon materials 18 , and other materials 19 . Our research team is continuously making great efforts on the porous organic polymers (POPs) self-supported single Rh active site catalysts (Rh–POPs) which present prodigious potential in heterogeneous hydroformylation due to the unique microenvironment of single dispersed Rh sites and stable phosphorous polymer frames 20 , 21 , 22 , 23 , 24 . In August 2020, the world’s first demonstration project of heterogeneous ethylene hydroformylation and further hydrogenated for the production of n-propanol (50,000 t/year), developed by the Dalian Institute of Chemical Physics of the Chinese Academy of Science, was proved successful with the adoption of single-site Rh–POPs catalyst in Zhejiang Province, China 3 .
Compared with the reactant of ethylene, precisely controlling the regioselectivity is the determinant in the process of promoting propylene hydroformylation 25 . Steric hindrance and electronic effects are recognized as critical factors affecting regioselectivity, which have been precisely studied in homogeneous hydroformylation with the natural property of a specific single active site 26 , 27 , 28 , 29 . However, the complex microenvironment of the active center on a solid surface makes it challenging to reveal the truly active intermediates in heterogeneous catalysis. Besides, the atomic understanding of the dynamic evolution of the single Rh active center under working conditions and its effect on regioselectivity are still unclear, leading to insufficient guidance for efficacious catalyst design.
In addition to focusing on the single metal active center, the coordinated ligands can also affect the catalyst activity. Particularly, the hemilabile ligands can be dissociated and re-coordinated at the metal center with open and closed state, which provide the space and driving force for the reactant adsorption, activation and reaction 30 , 31 , 32 , 33 . The concept of hemilability has been established in homogeneous catalysis, but rarely discussed in heterogeneous catalysis probably due to the competition between the metal-support coordination and the complex microenvironment on the solid support. Hence, there is a certain gap to unify the hemilability in both homogeneous and heterogeneous catalysis 34 . Metal-supported POPs materials have the characteristics of single metal active site and flexible supported framework, which is very suitable for exploring the hemilability in heterogeneous catalysis. In our Rh–POPs catalyst system for hydroformylation, the coordination and activation of reactant correspond to the hemilabile ligands dissociated and re-coordinated at the isolated Rh active center and the surrounding microenvironment, which significantly influences the regioselectivity of products. Therefore, the exploration of efficient methods to accurately elucidate this dynamic reaction process is of great significance for the understanding of the reaction mechanism.
Herein, a series of Rh–POPs catalysts were elaborately prepared by controlling the Rh contents and then employed in the propylene hydroformylation. Combining multiple techniques such as HAADF-STEM, XAFS, FT-IR, multi-dimensional correlation Solid-state NMR and DFT calculations, two single Rh active sites with distinct P–Rh–P bite angles were unambiguously identified. The experimental evidence of the dynamic evolution of hemilabile ligands dissociation and re-coordination at a single Rh active center were proved by in situ XAFS, FT-IR spectroscopy, and quasi-in situ NMR experiments (Fig. 1 ). Furthermore, the reaction mechanism was identified by DFT theoretical calculation, and revealed that the hemilabile coordination could effectively change the electronic structure and steric hindrance of the Rh active center to achieve adjustable regioselectivity, which extended deeper understanding of hemilabile coordination in heterogeneous catalysis and provided more possibilities for the industrialization of heterogeneous propylene hydroformylation.
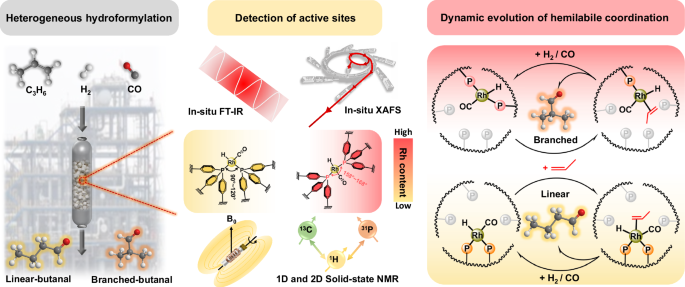
Multiple techniques such as in situ XAFS and FT-IR combined with Solid-state NMR to unravel the reaction mechanism of propylene hydroformylation, from accurate identification to dynamic evolution of the active center during the reaction process over Rh–POPs catalysts. Direct experimental observation revealed how hemilabile PPh 3 -frame ligands dissociate and re-coordinate accompany the propylene reaction and desorption, then influence the regioselectivity in heterogeneous propylene hydroformylation.
Construction of Rh–POPs catalysts
The Rh–POPs samples were synthesized using the impregnation method by introducing rhodium precursor on the POPs–PPh 3 support which referred to our previous work 20 , 24 . Multiple characterizations including ICP, XRD, TG, N 2 sorption, and SEM images indicate the Rh–POPs catalysts processing high specific surface area, hierarchical porosity, and relative good thermostability (Supplementary Figs. 1 – 5 and Supplementary Tables 1 and 2 ). High-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) and the corresponding STEM-energy dispersive spectroscopy (STEM-EDS) elemental mapping images show that isolated individual Rh atoms are uniformly dispersed within the POPs framework without any nanoparticles or clusters of Rh species with the Rh loading from 0.25 to 5 wt% (Fig. 2a–c and Supplementary Figs. 6 and 7 ).
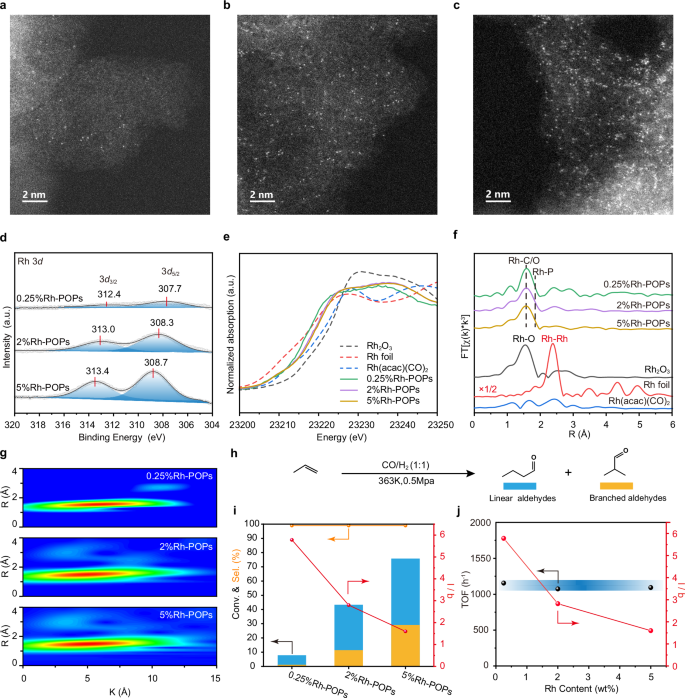
HAADF-STEM images of a 0.25%Rh–POPs, b 2%Rh–POPs, c 5%Rh–POPs; d Rh 3 d XPS of Rh–POPs catalysts; e Normalized Rh K-edge XANEs spectra, f FT k 3 -weighted EXAFS spectra in R-space, and g Wavelet Transforms EXAFS (WT-EXAFS) spectra of Rh–POPs catalysts with different Rh loadings; Reaction pathway ( h ) and catalytic performance ( i , j ) of propylene hydroformylation over Rh–POPs catalysts, Conv. represented the conversion of propylene, Sel. represented the selectivity of product aldehydes, including linear aldehydes (blue color) and branched aldehydes (yellow color), l/b represented the ratio of linear and branched aldehydes. TOF was calculated with the lower propylene conversion (<10%) conditions, detailed reaction conditions are shown in Supplementary Table 4 .
X-ray photoelectron spectroscopy (XPS) was utilized to investigate the electronic states and coordination environment of the unique Rh single sites of Rh–POPs (Fig. 2d and Supplementary Fig. 8 ). The spectrum of POPs–PPh 3 support shows two peaks at 132.0 and 130.2 eV, corresponding to P 2 p 3/2 of the oxidized and uncoordinated phosphine species, respectively (Supplementary Fig. 8 ) 35 . With the introduction of Rh species, a new signal appears at 131.5 eV, which can represent the P 2 p 3/2 of the phosphine ligands that coordinated to Rh atoms. The positive shift from 130.2 to 131.5 eV of P 2 p 3/2 can be ascribed to the lone pair electrons filling the empty orbital of the Rh atom to form the Rh–P coordination bonds, decreasing the electron density of uncoordinated phosphine. The Rh 3 d spectrum of every Rh–POPs sample represents two peaks, which are attributed to the 3 d 5/2 and 3 d 3/2 of Rh + , suggesting the Rh species existed in the form of +1 oxidation state for all the catalysts (Fig. 2d ) 24 . As the Rh contents increased from 0.25 wt% to 5 wt%, the binding energy (B.E.) of Rh 3 d 5/2 shifts to the higher energy (from 307.7 to 308.7 eV), indicating that the density of the Rh electronic states decreases along with the increase of Rh loading.
The Rh K-edge X-ray absorption near edge structure (XANES), extended X-ray absorption fine structure (EXAFS) and Wavelet Transforms EXAFS (WT-EXAFS) spectroscopy were employed to further determine the local coordination and electronic structures of the Rh–POPs samples (Fig. 2e–g ). The Rh K-edge XANES spectra indicate that all POPs support Rh species process a higher oxidation state than the Rh foil and lower than that of the Rh 2 O 3 , which are very close to the Rh(acac)(CO) 2 . According to the rising edge overlapping that of Rh(acac)(CO) 2 , Rh species in all three samples are mainly maintained +1 oxidation state, which is in good consistent with XPS characterization 36 . In the EXAFS spectra, the major scattering peak at around 1.5–1.6 Å is ascribed to the first coordination shell of Rh–C/O and the shoulder peak appeared at about 1.9 Å is ascribed to the first coordination shell of Rh–P 19 . The Rh–Rh shell at 2.4 Å cannot be found in the EXAFS spectra and WT-EXAFS spectra of all the Rh–POPs samples, in good consistent with the HAADF-STEM results, indicating that the phosphine ligand of POPs supports could provide a unique coordination environment to immobilize the Rh species as single sites even the Rh loading up to 5 wt%.
The fitted EXAFS results of Rh–POPs in the first shell are shown in Supplementary Fig. 9 and Supplementary Table 3 . The 0.25%Rh–POPs shows a well-defined structure with a 3.0 coordination number (CN) of Rh–P (2.29 Å) and 2.0 CN of Rh–C/O (2.03 Å), suggesting the P atoms of the POPs framework completely replaced the CO group in the precursor Rh(acac)(CO) 2 to form the active center at low Rh content. However, the coordination states of the 2% and 5%Rh–POPs samples are significantly different from the 0.25%Rh–POPs sample with a decreased CN (2.0) of Rh–P and increased CN (3.0) of Rh–C/O. This indicates that a number of the Rh–CO in the Rh–POPs with higher Rh content are retained during the impregnation process. The reduced Rh–P coordination number and the emerging Rh–C coordination number induce the d electrons of the Rh active center to fill into the 2π anti-bonding orbital of CO, resulting in a decrease in the electron cloud density.
The catalytic performance of propylene hydroformylation over Rh–POPs catalysts was tested in a fixed-bed reactor with the 0.5 MPa reactant gas at 363 K. The propylene conversion, butyraldehyde selectivity, the ratio of linear and branched product (l/b), and TOF value were evaluated (Fig. 2h–j and Supplementary Table 4 ). All three samples represent perfect butyraldehyde selectivity (>99%), and the propylene conversion is increased from 7.86 to 75.8% with the Rh content from 0.25 to 5 wt%. However, it is notable that the l/b ratio decreases continuously from 5.78 to 1.6. To exclude the effects of secondary reaction and diffusion, we tested these three samples at lower conversion (<10%, Fig. 2h ). All the samples possess a similar TOF value of ~1150 h −1 but a decreased l/b ratio with the increased Rh contents. The noticeable distinction in regioselectivity is most likely owing to the peculiar microenvironment of the single Rh active site.
Precise structures of the active centers
Solid-state nuclear magnetic resonance (ssNMR) has the unique advantage of characterizing the microstructure of the active center. The framework of POPs is mainly composed of triphenylphosphine polymer, and 1 H- 13 C Cross-Polarization (CP) MAS NMR could provide insight into the microenvironment of the Rh–POPs before and after syngas treatment (Fig. 3a ). The main peaks appear in the range from 126 to 146 ppm, which is attributed to the aromatic carbons of PPh 3 -framework. The signals appeared at 40 and 45 ppm are ascribed to the polymerized vinyl groups, and the peak at 112 ppm is assigned to unpolymerized residual vinyl functional groups 23 . Before the syngas treatment, with the increase of Rh loading, three signals are gradually highlighted at 31, 100, and 187 ppm, which are attributed to the signal of acetylacetone on the Rh (I) precursor 37 . After activation under syngas atmosphere, two new signals can be observed at 201 and 199 ppm, which are the characteristic signals of the linear and branched aldehyde groups, respectively. The intensity of the residual unpolymerized vinyl groups corresponding to 113 ppm decreases to a certain extent, demonstrating the hydroformylation of the unpolymerized vinyl functional group on the POPs support. An extraordinary signal appeared at around -10 ppm in the 1 H MAS NMR of 5%Rh–POPs is unquestionably ascribed to the proton bonded to the Rh active center, the so-called Rh–H species (Supplementary Fig. 10 ) 38 , 39 . Combined with the decrease of the 13 C NMR signal at 31, 100, and 187 ppm, it can be concluded that the acetylacetone are dissociated accompany by the formation of Rh–H bond during the activation process. Simultaneously emerged signals at 15, 28, 45, and 52 ppm of the 13 C MAS NMR confirmed the formation of aldehyde chains after hydroformylation of vinyl functional groups, indicating high hydroformylation activity of the single Rh active site (Supplementary Fig. 11 ). The signals of tetrahydrofuran appeared at 26 and 67 ppm indicate that some solvent remains in the catalysts. The detailed structures were recognized by complementary 2D heteronuclear correlation spectroscopy, such as 13 C{ 1 H} and 1 H{ 13 C} HETCOR MAS NMR spectra (Supplementary Figs. 12 and 13 ), and an overview of 13 C species is displayed in the Supplementary Fig. 14 .
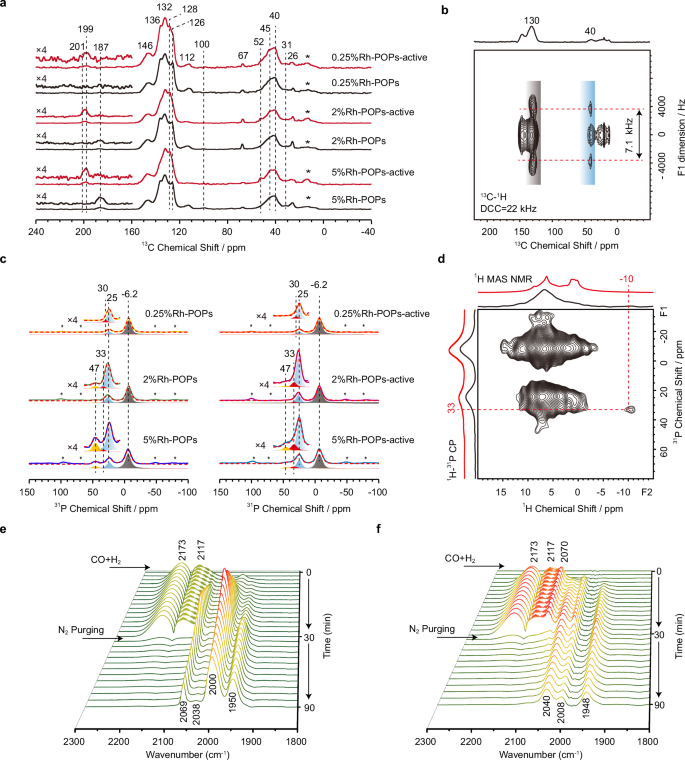
a 1 H- 13 C CP MAS NMR spectra of Rh–POPs catalysts before and after activation by syngas. b 13 C{ 1 H} 2D R-SLF NMR spectra of 5%Rh–POPs-active sample. c 31 P MAS NMR spectra and the fitness of Rh–POPs catalysts before and after activation by syngas. d 1 H{ 31 P} HETCOR MAS NMR spectrum of 5%Rh–POPs-active catalyst, the corresponding 1 H MAS NMR and 1 H- 31 P CP MAS NMR are displayed on the top and left of the 2D spectrum. In situ time-resolved FT-IR spectroscopy study of e 0.25%Rh–POPs and f 5%Rh–POPs in syngas feeding at 363 K and purging with N 2 subsequently.
13 C{ 1 H} 2D separated local field (R-SLF) NMR experiments were performed to disclose the microenvironment of a series of Rh–POPs-active samples (Fig. 3b and Supplementary Fig. 15 ). The residual 13 C– 1 H dipolar coupling can be used as an index to evaluate the motility of molecules or intramolecular segments of the POPs framework. The 13 C– 1 H dipole coupling of CH and CH 2 groups in rigid molecules is ~22 kHz. If the molecular or carbon chain segment is relatively mobile, the molecular motion will average the residual dipole coupling, resulting in the final measured 13 C– 1 H dipole coupling less than 22 kHz 40 . Two peaks appear around 130 ppm and 40 ppm in the F2 dimension of the spectra, which can be attributed to the CH species in the aromatic hydrocarbon and polyvinyl segment of the POPs framework, respectively. The residual 13 C– 1 H dipole coupling of all the samples maintains at 22 kHz with the increase of Rh doping from 0.25 to 5 wt%, indicating the robust structural stability under syngas activation.
Resolving the local structure of the active site by detecting the signal of P is undoubtedly very straightforward because Rh and P are directly coordinated. Herein, 31 P MAS NMR and 1 H– 31 P CP MAS NMR spectra are used to monitor the precise structure and evolution of the active sites before and after activation of the catalysts (Fig. 3c and Supplementary Fig. 16 ). There are two obvious peaks at −6 and 25 ppm of all the samples, representing uncoordinated P atoms and slightly oxidized P = O species of the POPs framework 21 . In the 0.25%Rh–POPs sample, a shoulder signal around 30 ppm appears at the lower field of the P = O peak, which is attributed to P atoms with multiple coordination bonds connected with the Rh atom 41 , 42 . As the content of Rh increases to 2 wt% and 5 wt%, a new signal appears at 47 ppm and the peak intensity increases with the Rh loading, which is attributed to Rh(acac)(CO)(PPh 3 -frame) 43 . All samples were inevitably mildly oxidized during syngas activation and subsequent NMR testing, resulting in a slight increase in the signal at 26 ppm. In the 0.25%Rh–POPs, the signal at 30 ppm corresponding to the Rh–P multi-coordination bonds keeps unchanged after activation, indicating superior structure stability. It is worth noting that in the remaining two samples, the spectra change dramatically before and after the syngas activation, with the signal at 47 ppm decreased accompanied by an increased peak at 33 ppm, indicating that the state of P that coordinated with Rh is changed from monophosphate to polyphosphate during this process. 2D 1 H{ 31 P} heteronuclear correlation spectroscopy was adopted to further assist the attribution of the 31 P NMR signal as shown in Fig. 3d . The arresting correlated signal at (−10, 33) of 5%Rh–POPs sample proves the spatial proximity of P species at 33 ppm and the proton of the Rh–H bond. The standard sample HRh(CO)(PPh 3 ) 3 was used as a reference and the analogous correlated signal proved the correctness of attribution that the 33 ppm is the P species directly bonded to the Rh atom (Supplementary Figs. 17 and 18 ).
For deeper insight into the coordination state of the single Rh active site and how the active center dynamically changes during activation through syngas, in situ time-resolved FT-IR spectroscopy was conducted (Fig. 3e, f and Supplementary Fig. 19 ). Two distinct absorption bands at 2173 and 2117 cm −1 , gradually increase with the injection of syngas and decrease until vanish with the purge of nitrogen, which is attributed to the CO gas. In the spectrum of 0.25%Rh–POPs (Fig. 3e ), the peak at 2069 cm −1 is ascribed to the ν(Rh–CO) of the HRh(CO)(PPh 3 -frame) 3 species 44 . The bands at 2000 and 1950 cm −1 are belonged to the ν(Rh–CO) stretching vibration of HRh(CO) 2 (PPh 3 -frame) 2 , and the remaining critical signal at 2038 cm −1 is ascribed to the stretching vibration of ν(Rh–H) species 24 , 29 , 45 . In the 5%Rh–POPs sample (Fig. 3f ), a peak attributed to the Rh–H species analogously appeared at 2040 cm −1 and this attribution can be proved by the H-D exchange experiments (Supplementary Fig. 20 ). The ν(Rh–CO) stretching vibrations of HRh(CO) 2 (PPh 3 -frame) 2 are appear at 2008 and 1948 cm −1 , representing a slight shift in wavenumber compared with the sample of 0.25%Rh–POPs (2000 and 1950 cm −1 ). This result indicates that the electron state of Rh is different in these two samples, which can affect the vibration wavenumber of Rh–CO species. The active center of the 2%Rh–POPs sample (Supplementary Fig. 19 ) shows a similar spectrum to that of the 5%Rh–POPs sample. Hence, the 0.25%Rh–POPs and 5%Rh–POPs were selected as representative samples for further comparison. It is worth noting that two penta-coordinate HRh(CO)(PPh 3 -frame) 3 and HRh(CO) 2 (PPh 3 -frame) 2 could be simultaneously observed in the IR spectrum, but in the real reaction process, a Rh–P or Rh–CO bond will be dissociated to form the tetradentate HRh(CO)(PPh 3 -frame) 2 species for receiving the olefin coordination during the hydroformylation reaction 20 , 39 , 46 . For better understanding, HRh(CO)(PPh 3 -frame) 2 is used to represent the real active center, in which the bite angle specifically refers to the P–Rh–P angle of this active center.
In the 5%Rh–POPs (Fig. 3f ), the Rh–H species appeared at 2040 cm −1 and the intensity is significantly stronger than that of the 0.25%Rh–POPs sample (Supplementary Fig. 21 ), which is in consistent with the 1 H MAS NMR (Supplementary Fig. 10 ), while the signal at −10 ppm cannot be observed in 0.25%Rh–POPs sample mainly because the content of Rh–H species in this sample is too low to detect by NMR. The signal at 2070 cm −1 gradually increase with the introduction of syngas but gradually disappear with the purge of N 2 , indicating the HRh(CO)(PPh 3 -frame) 3 species cannot be stabilized in this sample. Meanwhile, after the purging of N 2 , the signal intensity at 2070 cm −1 of the 5%Rh–POPs is much lower than that of the 0.25%Rh–POPs. This illustrates that the bite angle of P–Rh–P of 5%Rh–POPs is larger than that of 0.25%Rh–POPs, and the large steric hindrance makes the third PPh 3 -frame difficult to coordinate with Rh.
The P–Rh–P bite angle of 0.25%Rh–POPs has been well demonstrated with the region between 90° and 120° that was referred to the homogeneous active center HRh(CO) 2 (PPh 3 ) 2 with ee (120°) and ea (90°) isomer in hydroformylation 20 , 23 , 24 . Therefore, we hypothesize that the bite angle could surpass the range of 90–120°, particularly with an increase in Rh content. To determine the possible range of P–Rh–P bite angles, DFT calculations were employed for detailed analysis (Fig. 4a, b , Supplementary Fig. 22 , and Supplementary Tables 5 and 6 ). The phosphine ligands in Rh–POPs materials are immobilized on the POPs framework, which are very different in homogeneous situations where the phosphine ligands exhibit high flexibility in solvents. Although the POPs framework possesses a certain level of flexibility, it restricts the range of movement for coordinated P ligands compared to that observed in a homogeneous phase. To streamline the computational model and make it closer to the real heterogeneous conditions, triphenylphosphine is selected to replace the PPh 3 -frame, and the three farthest protons of the three benzene rings opposite to the P atom are immobilized to restrict the mobility of coordinated P atoms. The geometric center of the plane created by three protons serves as a representation of the coordination site for the P ligand, while the spatial distance between the two geometric centers is designated as the ligand coordination distance (Fig. 4a ). Hence the different bite angles and P–P distance are determined by adjusting the ligand coordination distance.
a Schematic diagram described the gravity distance of the single Rh active center. b Optimized structures of the Rh active center by regulating gravity distance and related DFT analysis for the relative energy and P–Rh–P bite angle, i – viii represent the structures with Rh–H and Rh–CO coordinated on the same side, corresponding i’ – viii’ represented the opposite side. c Dynamic evolving trajectories of Rh active center before and after activation by syngas. d Schematic illustration of the probable Rh positions under the certain circumstance of lower and higher Rh content, for ease of viewing, Rh–H and Rh–CO are not drawn in the figure.
In the case of only two PPh 3 coordinated with Rh atom, a series of possible stabilized Rh–POPs structures were optimized by regulating the ligand coordination distance from 9.9 Å to 12.3 Å (Supplementary Fig. 22 ). The range of P–Rh–P angles can be obtained from 103.2° to 175°, correspondingly the P–P distance from 3.41 to 5.01 Å. It is interesting to find that the angles show a completely different tendency in different regions as the ligand coordination distance increased from 9.9 Å to 12.3 Å. In the range from 9.9 Å to 10.8 Å, the angles increase linearly from 103.2° to 124.7°. However, the ligand coordination distance raised from 10.8 Å to 11.1 Å, the angle changes with a huge jump from 124.7° to 168.7°. As the ligand coordination distance continues to increase from 11.1 Å to 12.3 Å, the angles increase linearly again from 168.7° to 175.0°. The notable difference observed in these two regions (below 124.7° and above 168.7°) likely stems from the variation in hybridization between rhodium d -orbital and phosphorus p -orbital, a phenomenon influenced by the compound’s geometry.
Due to the HRh(CO)(PPh 3 -frame) 2 being the actual active center, Rh–H and Rh–CO were also taken into account as shown in Fig. 4b . The Rh–H and Rh–CO could be coordinated on the same side or opposite side, which also affects the P–Rh–P bite angle. In the range of ligand coordination distance from 9.9 Å to 10.4 Å, the Rh–H and Rh–CO are more inclined to coordinate on the same side, with the P–Rh–P bite angles between 95.4° and 104.1°. As the ligand coordination distance increases from 10.8 Å to 12.1 Å, it can be found that the active center structures formed by the opposite side coordination of Rh–H and Rh–CO have the lower energy, and the bite angel is in a certain range between 158.3° and 168.1°. Based on the above theoretical calculation, we can infer that two distinct active centers could exist with a discrete P–Rh–P angle range. Therefore, compared with the 0.25%Rh–POPs sample with the bite angel between 90° and 120°, we speculate that the enlarged bite angle may exist in the range from 158° to 168° with the increase of Rh content to 5 wt%.
The possible step-by-step evolution of the Rh–POPs active centers based on experiments and calculations are depicted in Fig. 4c . In the process of synthesis of Rh–POPs catalyst by impregnation method of Rh(acac)(CO) 2 precursor on the POPs framework, Rh(acac)(PPh 3 -frame) 2 (so-called Rh–POPs) is easily formed with the lower Rh content (0.25 wt%), then convert to HRh(CO)(PPh 3 -frame) 3 and HRh(CO) 2 (PPh 3 -frame) 2 species (so-called Rh–POPs-active) during the process of syngas activation in which these two species could be transformed each other under the CO atmosphere. Subsequently, a Rh–P or Rh–CO bond dissociated accompanied by the formation of HRh(CO)(PPh 3 -frame) 2 species with the P–Rh–P bite angle between 90° and 120° for further hydroformylation reaction. When the Rh loading increased to 5 wt%, Rh(acac)(CO)(PPh 3 -frame) species were formed. Then the acetylacetone is removed under the treatment of syngas, along with the formation of the Rh–H bond. Besides, Rh forms a new coordination bond with the neighboring PPh 3 -frame to generate HRh(CO) 2 (PPh 3 -frame) 2 species. Finally, a CO is dissociated to form the truly active center HRh(CO)(PPh 3 -frame) 2 with the P–Rh–P bite angle between 158° and 168°.
We have hypothesized the possible reasons for the distinct active centers caused by different Rh loading as shown in Fig. 4d . In the case of lower Rh content, Rh (I) precursor is more inclined to locate in the rich P area during the impregnation process and the P atom on the POPs framework completely replaces all the CO of the precursor Rh(acac)(CO) 2 to form Rh(acac)(PPh 3 -frame) 2 . After activation by syngas, Rh–H bond formation is accompanied by the removal of acetylacetone. At the same time, one of the surrounding superfluous P atoms will coordinate with a single Rh active center to form the HRh(CO)(PPh 3 -frame) 3 species. With the increase of Rh loading, the Rh (I) precursor has to settle in the region of lower P concentration. Due to the steric hindrance of acetylacetone, the P atom only replaces one of the CO groups to form Rh(acac)(CO)(PPh 3 -frame) species. With the activation of syngas, Rh will coordinate with the surrounding P atom after acetylacetone desorption. The lower p concentration and the large bite angle of P–Rh–P in the range from 158° to 168° make it difficult for a third P to coordinate with the Rh active center.
Dynamic evolution of hemilabile coordination
The above characterizations revealed the microstructure of the truly active center HRh(CO)(PPh 3 -frame) 2 with distinct P–Rh–P bite angles under different rhodium content. To understand how single Rh active centers regulate the distribution of product aldehydes, a variety of characterizations such as in situ time-resolved XAFS and FT-IR, combined with quasi-in situ NMR have been used to explore the dynamic changes of active centers and coordinated species (Fig. 5a–e ). In situ XAFS were used to identify the change of Rh valence state and electron cloud density of the 5%Rh–POPs during the reaction process as shown in Fig. 5a . It can be seen from the time-resolved spectra with the injection of reaction gas, a drop in white line intensity at 23,247 eV indicates that the coordination state of Rh changes dynamically during the reaction process 13 . The adsorption edge position at around 23,220 eV slightly shifts to the lower energy, indicating a gradual decrease in the electron cloud density of the Rh active center and this maybe ascribe to the CO replaces the coordinated PPh 3 -frame in the reaction process 47 . However, the slight change in the electron cloud density is insufficient to affect the valence state of Rh, suggesting that the valence state of Rh is almost unchanged during the whole reaction process.
a In situ time-resolved Rh K-edge XAFS spectra of 5%Rh–POPs sample treated by mixture reactant (C 3 H 6 /CO/H 2 = 1:1:1) for 20 min at 363 K. b In situ time-resolved FT-IR spectroscopy study of 5%Rh–POPs-active in mixture reactant (C 3 H 6 /CO/H 2 = 1:1:1) feeding for 30 min at 363 K. c 31 P MAS NMR spectra and the fitness of sample S I , S II , and S III (sample S I : 5%Rh–POPs-active; sample S II : sample S I treated with C 3 H 6 at 363 K; sample S III : sample S II treated with syngas at 363 K). d Comparison of 1 H{ 31 P} HETCOR MAS NMR spectrum of S II and S III , the corresponding 1 H MAS NMR and 1 H- 31 P CP MAS NMR are displayed on the top and left of the 2D spectrum (black line: sample S II , red line: sample S III ). e 1 H- 13 C CP MAS NMR spectra of sample S I , S II , and S III . f Schematic diagram described the dynamic evolution of the active center and hemilabile coordination under reaction conditions.
In situ time-resolved FT-IR spectroscopy was used to intensively explore the reaction process of 5%Rh–POPs-active sample with the reactants (C 3 H 6 /CO/H 2 = 1:1:1) and then purging with N 2 (Fig. 5b , Supplementary Figs. 23 and 24 , and Supplementary Table 7 ). The peaks at 1664 cm −1 and 1642 cm −1 are attributed to the ν(C = C) of C 3 H 6 . The peaks at 991 cm −1 and 912 cm −1 are ascribed to the ν(C–H) out-of-plane bending vibration on unsaturated carbon atoms of propylene 48 , 49 . The antisymmetric bending vibrations of ν(−CH 2 −) and ν(−CH 3 ) are presented at 1442 cm −1 and 1473 cm −1 , respectively. The multi-peaks between 2880 cm −1 and 3104 cm −1 are ascribed to the symmetrical and antisymmetric stretching ν(C–H) vibration of −CH 2 − and −CH 3 groups. The characteristic adsorption peak at 1728 cm −1 is attributed to the stretching vibration of ν(C = O) in the product aldehyde, and the corresponding ν(C–H) bending and stretching vibration of aldehyde group appeared at 2714 cm −1 and 2811 cm −1 , respectively 50 . These three characteristic peaks gradually increase with the introduction of reactant, accompanied by the ν(Rh–H) and ν(Rh–CO) species, indicating the occurrences of hydroformylation at the active center.
In addition to the above in situ characterization techniques, quasi-in situ ssNMR method was used to monitor the dynamic changes of the coordinated ligands and reaction active intermediates. The 5%Rh–POPs-active sample (labeled as “ S I ”) was stepwise treated with C 3 H 6 (labeled as “ S II ”) and syngas (labeled as “ S III ”) at reaction conditions and then quenched by liquid nitrogen for subsequent 31 P and 13 C NMR experiments (Fig. 5c–e ). The truly active center of the 5%Rh–POPs-active sample has been verified with the specific structure of HRh(CO)(PPh 3 -frame) 2, and the bite angle of P–Rh–P is between 158° and 168°. With the introduction of propylene, it is interesting to find that the signal of Rh connected with gemini PPh 3 -frame at 33 ppm significantly decreases, corresponding to the prominent increase of characteristic signal of Rh coordinated with mono PPh 3 -frame at 47 ppm. This suggests the coordination ability of propylene to Rh is stronger than that of PPh 3 -frame, which results in one Rh–P coordination bond dissociation in the coordination process. As to sample S II , it can be seen the signal at 47 ppm reduces accompanied by the signal at 33 ppm increasing with the introduction of syngas. The compared 1 H{ 31 P} HETCOR spectra of sample S II and S III were also adopted to further explain this attribution as shown in Fig. 5d . The cross peak of sample S II at (6.8, 47) disappears after the syngas introduction, along with the cross peak emerged at (−10, 33), indicating the syngas processing hydroformylation reaction with the coordinated propylene. With the Rh–H species formation and the product aldehyde desorption, the coordinate state of Rh will change from a single PPh 3 -frame to a gemini PPh 3 -frame. 1 H- 13 C CP and 13 C MAS NMR spectra were adopted to further explore the intermediates in the reaction process (Fig. 5e and Supplementary Fig. 25 ). As compared with the initial 5%Rh–POPs-active sample, the signals at 18, 115, and 137 ppm generated with the introduction of propylene are attributed to the −CH 3 , −CH = , and =CH 2 of propylene, respectively. Two notable signals at 184 and 187 ppm are ascribed to the C = O species of the Rh-acyl group, indicating that propylene coordinated on the active center and subsequently interacted with the Rh–H bond to form Rh-alkyl, then CO inserted into the Rh-alkyl group to form Rh-acyl species. Based on this state (sample S II ) with the continuous injecting of syngas, the signals at 115, 184, and 187 ppm disappeared, indicating that the hydroformylation reaction continued and the coordinated propylene was completely consumed. After the hydroformylation reaction, some new signals appear mainly in the range of 13 to 45 ppm (13, 16, 40, and 45 ppm), which are attributed to the product of butyraldehyde. These signals are sharp in the 13 C CP and MAS NMR spectra, indicating the residual butyraldehyde has strong mobility and can be easily desorbed from the Rh–POPs framework.
Based on the above characterizations and analysis, the dynamic dissociation and re-coordination between the metal center and coordinated ligands can be demonstrated during the adsorption, reaction, and desorption of guest molecules in the reaction process, which can be the so-called hemilability. Hemilability is an important concept in homogeneous catalysis, that is, the activation of reactants and the formation of products can occur simultaneously through the reversible opening and closing of the coordination state between metal and ligand (Supplementary Fig. 26 ). However, the dynamic change of this coordination state and its effect on the reaction is rarely discussed in heterogeneous catalysis. Meanwhile, the complexity of the active centers in heterogeneous catalysis makes it more difficult to give explicit experimental evidence for hemilabile coordination. Herein, the Rh–POPs catalyst system contains a well-defined structure with flexible framework and dynamic evolution active sites, which can be used as an ideal model to understand the hemilabile coordination property. Combined with the deep understanding of the microenvironment of the truly active center and the capture of the active intermediate under in situ conditions, we use schematic diagram to describe the dynamic evolution between the active center and hemilabile coordination in the process of guest molecules adsorption, activation, formation of intermediates and finally desorption (Fig. 5f ).
In the 0.25%Rh–POPs sample with lower Rh content, the bite angle of metal center and the coordinated ligands is between 90° and 120°, which makes the ligands connected to the metal center more stable, causing reactant molecule coordination and activation without breaking the metal–ligand coordination bond, as proved by in situ time-resolved FT-IR spectroscopy and 31 P MAS NMR (Supplementary Figs. 27 and 28 ). However, in the 5%Rh–POPs sample with higher Rh content, the bite angle is between 158° and 168°, and thereby makes the ligand hemilabile. With the coordination and activation of guest molecules, the coordination bond between a certain metal and the ligand will be broken into an open state, and then the hemilabile ligand will re-coordinate with the metal center and become to a closed state, accompanied by the generation and desorption of products.
Mechanism of the propylene hydroformylation over Rh–POPs
Through the comprehensive understanding of hemilability in heterogeneous catalysis and the precise analysis of the microenvironment of the active center, the reaction mechanism of propylene hydroformylation is proposed (Fig. 6a ). DFT calculation was also used to confirm the feasibility of the reaction path (Fig. 6b–d ). In the process of propylene hydroformylation catalyzed by Rh–POPs material with a lower Rh content such as 0.25 wt%, one PPh 3 -frame or CO of the HRh(CO)(PPh 3 -frame) 3 and HRh(CO) 2 (PPh 3 -frame) 2 ( I and I rev in Fig. 6a ) will be dissociated, resulting in a real active center of HRh(CO)(PPh 3 -frame) 2 with the P–Rh–P bite angle between 90° and 120° ( II in Fig. 6a ). In the higher Rh content such as 2 wt% and 5 wt%, one CO of the HRh(CO) 2 (PPh 3 -frame) 2 ( I’ in Fig. 6a ) will be dissociated to result in HRh(CO)(PPh 3 -frame) 2 with an enlarged P–Rh–P bite angle from 158° to 168° ( II’ in Fig. 6a ). Two typical models with the ligand coordination distance of 10.1 Å and 12.1 Å were chosen to represent the truly active center II and II’ of which the P–Rh–P bite angle at 100° and 168°, respectively.
a Proposed reaction cycle of the propylene hydroformylation over Rh active centers with lower and higher Rh content. b Optimized structures of the active center II (top) and II’ (bottom) with the coordination of propylene. c 2D color distribution of the localized-orbital locator (LOL) projection along the P–Rh–P plane of the II (top) and II’ (bottom). d Relative energy between linear and branched products of the Rh active center with lower and higher Rh contents following the reaction route of ( a ).
Prior to the coordinating with propylene, the Rh centers in the intermediate structures maintain a tetrahedral coordination configuration (Supplementary Fig. 29 ). When the hydroformylation reaction starts, propylene is activated by filling π electrons into the Rh empty d -orbital, and the corresponding optimized structures are shown in Fig. 6b . As proposed in the mechanism outlined in Fig. 6a , the propylene will be coordinated at the active center II to form a penta-coordinated intermediate ( III in Fig. 6a ) and the Rh still coordinated with two P atoms throughout the process, in which one Rh–P bond is stretched from 2.35 to 2.41 Å, while another Rh–P bond is remaining with the bond length of 2.41 Å. However, in the process of coordination and activation of propylene on the active center II’ , the hemilabile coordination phosphine ligand will be dissociated from the single Rh active center to form a tetra-coordinate intermediate ( III’ in Fig. 6a ), resulting in the coordination state of Rh from bisphosphate ligand to monophosphate ligand with the open state. The DFT calculation shows that the distance of one Rh–P bond is extended from 2.44 to 3.55 Å, which is much longer than that of one Rh–P bond, indicating a Rh–P bond fracturing during the coordination of propylene, meanwhile the distance of residual Rh–P bond is shortened from 2.44 to 2.34 Å.
In order to gain a deeper understanding of the differential molecular coordination following propylene coordination in the two scenarios. A bond characteristic relying on the kinetic-energy density, known as the localized-orbital locator (LOL), was employed for characterizing the chemical bond nature of the structures (Fig. 6c ). One can clearly see that the localization of orbitals between the Rh and P bonds is more pronounced near P in the structure II’ ; hence, the bonding between Rh and P in structure II’ is weaker as compared to that in structure II . In addition, the average Mayer bond order of the Rh–P in II’ was 1.14 which is smaller than that of II with the value of 1.32, indicating that the degree of the electron cloud between Rh and P atom of II’ is less overlapping. This is also the reason why, after propylene coordinated in structure II’ , one of Rh–P bond breaks, leading to a stable tetrahedral coordination structure formation.
Subsequently, the different configurations of Rh-alkyl are formed when the coordinated propylene inserted into the Rh–H bond, which determines whether the final aldehydes are linear or branched. It is more inclined to form the linear Rh–C 3 H 7 active intermediate ( V in Fig. 6a ) due to the crowded steric hindrance provided by the gemini PPh 3 –ligands with lower Rh content. However, the branched Rh–C 3 H 7 ( V’ in Fig. 6a ) is easier to form due to the large reaction space provided by the monophosphate-coordinated ligand with higher Rh content. The energy differences between linear and branched intermediates were also calculated by the DFT calculation (Fig. 6d ). It can be seen from the relative energy diagram that the energy of the products formed by linear aldehydes is lower, so the l/b ratio of the products will be larger than 1, which is also in consistent with the results of the hydroformylation of propylene (Fig. 2i ). At lower Rh content, the energy difference between the linear and branched product is 0.72 eV, which is much higher than that of higher Rh content sample (0.23 eV). This indicates that the possibility of the branched product will increase accompanied by the Rh loading, which is also in consistent with the results that the l/b ratio of the 0.25%Rh–POPs and 5%Rh–POPs are 5.78 and 1.6, respectively.
Following the coordination of CO, it is inserted into the Rh–C 3 H 7 group to form (C 3 H 7 CO)Rh(CO)(PPh 3 -frame) 2 active species ( VI and VI’ in Fig. 6a ) through carbonylation. Finally, linear and branched butyraldehyde is eliminated through a hydrogenation reaction, and the catalyst returns to the initial HRh(CO)(PPh 3 -frame) 2 state ( II and II’ in Fig. 6a ) to complete the catalytic cycle. In the whole catalytic cycle, Rh keeps coordinated with the gemini PPh 3 -frame at lower Rh content, which makes the higher l/b ratio of the aldehydes. In the higher Rh content, the dissociation and re-coordination between the hemilabile coordination PPh 3 -frame ligands and the single Rh active center are the keys to the formation of branched aldehydes, which is also the reason for the decline of the l/b ratio.
In summary, we have demonstrated that Rh species present as single sites in a series of well-defined Rh–POPs catalysts through HAADF-STEM, XAFS, and XPS spectroscopy. The microenvironment of genuine active sites from Rh precursor impregnated on POPs support with the activation by syngas were analyzed step by step via FT-IR, advanced ssNMR techniques and DFT calculations. These characteristic HRh(CO)(PPh 3 -frame) 2 active centers exhibited distinct P–Rh–P bite angles with Rh loading from low to high, which represented adjustable regioselectivity in the hydroformylation reaction of propylene.
The dynamic evolution of the Rh active center and hemilabile coordination ligands of the higher Rh content sample under reaction conditions were elaborately interpreted by in situ time-resolved XAFS, FT-IR, and quasi-in situ ssNMR spectroscopy. First, the Rh–H bond was formed after syngas activation, then the propylene molecule was coordinated and activated accompanied by the broken hemilabile coordination Rh–P bond with an open state. Due to the large space provided by the open state, the smaller steric hindrance makes it easier to form isomeric products by inserting C 3 H 6 into the Rh–H bond to form branched Rh–C 3 H 7 species. Subsequently, CO carbonylated into branched Rh–C 3 H 7 to form Rh-acyl groups. Finally, branched butyraldehyde was produced and desorbed by hydrogenation accompanied by the Rh active center re-bonding with the hemilabile PPh 3 -frame ligands. The feasibility of the reaction path was proved by DFT theoretical calculation, and revealed the hemilabile coordination bond could effectively change the electronic structure of the Rh active center to regulate the regioselectivity of product aldehydes.
This experimental evidence exhaustively illustrated the dynamic evolution of the dissociation and re-coordination between hemilabile ligands and a single metal active site in heterogeneous hydroformylation reactions. Introduction, understanding, and application of the hemilability effects into the heterogeneous catalysis, which is an effective way to regulate catalyst reactivity in homogeneous, can provide a new perspective for high-efficiency heterogeneous catalyst design and offer an important guidance for the industrialization of propylene hydroformylation.
Sample preparation
The preparation of POPs: a tri-vinyl functionalized triphenylphosphine (3V-PPh 3 ) monomer was synthesized with the reaction between PCl 3 and (4-vinyl phenyl) magnesium bromide solution, then a saturated NH 4 Cl aqueous solution was added. The organic phase was extracted with ethyl acetate and then dried with MgSO 4 . After being filtered and purified by silica gel chromatography, the monomer was obtained. POPs were synthesized from the polymerization of the 3V-PPh 3 monomer under solvothermal conditions. 1.0 g of monomer was dissolved in 10 ml of THF, followed by the addition of 25 mg of azobisisobutyronitrile (AIBN). The mixture was transferred into an autoclave at 373 K for 24 h. After evaporation of THF under vacuum, the POPs support was obtained.
The preparation of Rh–POPs: Rh(acac)(CO) 2 was dissolved in 30 ml THF in a three-necked round bottom flask under an argon atmosphere, followed by a 30 min stirring to obtain a homogeneous solution. Then the POPs support was added to the round bottom flask and the obtained Rh mixture was stirred under an argon atmosphere at room temperature for another 24 h. The Rh–POPs catalyst was obtained by filtrating, washing with THF (70 ml), and drying under vacuum at 338 K. The theoretical loading of Rh metal was 0.25%, 2%, and 5%, and the actual Rh contents in the Rh–POPs were measured by the ICP method which was shown in Supplementary Table 1 . The measured results are almost in consistent with the theoretical Rh loading, hence the corresponding samples were named 0.25%Rh–POPs, 2%Rh–POPs, and 5%Rh–POPs, respectively.
Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a PANalytical X’Pert PRO X-ray diffractometer with Cu Kα radiation ( λ = 1.5045 Å) preparation at 40 kV and 40 mA.
High-Angle Annular Dark Field-Scanning Transmission Electron Microscopy (HAADF-STEM) and STEM-energy dispersive spectroscopy (STEM-EDS) elemental mapping images were recorded on a JEM-ARM200F instrument (JEOL) at 200 kV. Scanning-electron-microscope (SEM) images were recorded on a JSM-7800F instrument (JEOL) at 20 kV.
Thermogravimetric analysis (TGA) was performed on a NETZSCH STA 449F3 thermal analyzer. The catalyst was heated from 313 K to 1123 K with a heating rate of 10 K/min in N 2 flowing (20 mL/min).
N 2 sorption measurements were conducted on a Quantachrome Autosorb-1 sorption analyzer. About 0.10 g sample was degassed at 393 K under vacuum for 12 h and then tested in liquid N 2 (77 K). The specific surface area was calculated by a Brunauer–Emmett–Teller (BET) method. The pore volume was obtained at the P/P 0 = 0.998. The specific surface area was calculated by a Non-Local Density Functional Theory (NLDFT) method.
X-ray photoelectron spectroscopy (XPS) experiments were performed on a Thermo Scientific ESCALAB 250Xi equipped with an Al Kα radiation (1486.6 eV) X-ray source. The binding energies were referenced to C 1 s (284.8 eV).
Inductively coupled plasma optical emission spectrometry (ICP-OES) was performed using a PerkinElmer ICP-OES 7300DV. The sample was dissolved by a mixture of H 2 O 2 and Aqua regia on an Anton Paar Multiwave 3000 microwave instrument and then tested with the ICP-OES model.
In situ diffuse reflection infrared Fourier transform spectroscopy (in situ DRIFTS) experiments were conducted on a Thermo Scientific iS50 FT-IR spectrometer equipped with a mercury–cadmium–telluride (MCT) detector. Firstly, the sample was heated to 363 K in a N 2 flow (30 mL/min) for 60 min. After the spectra were stable, the background was collected. Then syngas (CO:H 2 = 1:1) was introduced for 30 min and the spectra were collected every minute. Later purged with N 2 for 60 min until the peak was unchanged and spectra were collected every 5 min. Ultimately, the mixture reactant (C 3 H 6 :CO:H 2 = 1:1:1) was introduced for 30 min, and the spectra were collected every minute, then purged with N 2 for 60 min until the peak unchanged and spectra were collected every 5 min. All the spectra were recorded between 800 cm −1 and 4000 cm −1 with 64 scans at a resolution of 4 cm −1 .
X-ray absorption fine structure (XAFS) experiments were performed at beamline BL14W1 of Shanghai Synchrotron Radiation Facility (SSRF, operated at 3.5 GeV with a maximum current of 200 mA, Rh K-edge). The sample was pelletized as disks of 13 mm diameter with 1 mm thickness using graphite powder as a binder. The data was recorded at room temperature and ambient atmosphere in the fluorescence mode equipped with an Electro–Lytle detector. In situ XAFS experiments were performed at beamline BL05U of SSRF. The sample was pressed into pellets with 2 mm thick and then placed into a stainless steel in situ cell which was surrounded by a heater and connected to a mass flow meter. The sample was heated at 363 K and then treated with the mixture reactant (C 3 H 6 : CO: H 2 = 1:1:1) for 20 min, and the spectrum was continuously collected during this process. The original EXAFS analyses were analyzed with the Artemis software package. The acquired EXAFS data were processed according to the standard procedures using the ATHENA module implemented in the IFEFIT software packages. The wavelet transform was carried out with the software module of FORTRAN.
1 H, 31 P, and 13 C Solid-state NMR (ssNMR) experiments were performed on a Bruker Avance NEO 400 spectrometer equipped with a 9.4 T and 89 mm wide-bore magnet using a 4.0 mm HX double resonances MAS probe with the corresponding Larmor frequencies of 400.2, 162, and 100.6 MHz, respectively. The chemical shifts were referenced to adamantane [δ( 1 H) = 1.74 ppm], 85% H 3 PO 4 [δ( 31 P) = 0 ppm], and the upfield methine peak of adamantane [δ( 13 C) = 29.5 ppm]. 1 H MAS NMR experiments were performed with a π/2 pulse width of 3.35 μs. 16 scans were accumulated with a spinning rate of 12 kHz and a recycle delay of 4 s. 13 C MAS NMR experiments were performed with a π/2 pulse width of 4.2 μs with 1 H decoupling, 4096 scans were accumulated with a spinning rate of 12 kHz and a recycle delay of 2 s. 1 H– 13 C Cross-Polarization (CP) MAS NMR experiments were performed with a contact time of 3 ms, 5120 scans were accumulated with a spinning rate of 12 kHz and a recycle delay of 2 s. 13 C{ 1 H} HETCOR were acquired with 512 scans for each of 20 experiments with a t 1 increment of 83.33 μs. 1 H{ 13 C} HETCOR were acquired with 64 scans for each of 30 experiments with a t 1 increment of 41.67 μs. 13 C{ 1 H} R-type RF irradiation and two-dimensional separated local field (2D R-SLF) were acquired with 160 scans for each of 60 experiments with a spinning rate of 11 kHz and a recycle delay of 3 s.
31 P MAS NMR experiments were performed with a π/2 pulse width of 3 μs with 1 H decoupling, 512 scans were accumulated with a spinning rate of 12 kHz and a recycle delay of 30 s. 1 H- 31 P CP MAS NMR experiments were performed with a contact time of 3 ms, 1024 scans were accumulated with a spinning rate of 12 kHz and a recycle delay of 2 s. 31 P{ 1 H} HETCOR were acquired with 512 scans for each of 20 experiments with a t 1 increment of 83.33 μs, the decoupling field of 62.5 kHz was applied during the acquisition time. 1 H{ 31 P} HETCOR were acquired with 256 scans for each of 30 experiments with a t 1 increment of 41.67 μs. The DMFIT 2015 software was used to simulate all the spectra and fit the peaks 51 . 31 P MAS NMR was fitted by Gauss/Lorenze linear.
Quasi-in situ NMR experiments were performed as follows. The Rh–POPs catalyst was placed into a stainless steel reaction tube and treated on a vacuum line at 363 K under high vacuum (<10 −5 Torr) for 6 h to remove the physically adsorbed organic solvents and gases. Keep the sample at 363 K and introduce 0.5 MPa syngas (CO:H 2 = 1:1) for 30 min and then quenched with liquid nitrogen rapidly. Excess of syngas was pumped away at RT and the corresponding sample was named Rh–POPs-active for further NMR experiments. After the NMR experiments, the Rh–POPs-active sample was treated on a vacuum line at room temperature and purged with 0.17 MPa C 3 H 6 , then raised the temperature to 363 K and reacted for 30 min. Quenched and pumped the excess gas after the reaction finished and then processed the NMR experiments. Finally, the sample from the previous step was further fed into the 0.5 MPa syngas for 30 min at 363 K and then quenched for NMR testing.
Catalytic reaction condition
The propylene hydroformylation reactions were performed in a stainless steel fixed-bed reactor at a pressure of 0.5 MPa. A certain amount of catalyst was filled in the center of the reactor. The upper and lower space was filled with quartz sand. After heating up to 363 K, the mixed reaction gas (C 3 H 6 /CO/H 2 = 1/1/1) was fed to the Rh–POPs catalyst with specific GHSV, detailed reaction conditions are shown in Supplementary Table 4 . The tailed gas was chilled at 278 K to collect the liquid product with the reaction lasted for 16 h. The incondensable gas products were detected by an online gas chromatograph (Agilent GC 7890B) equipped with a TCD detector and a PLOT-Q capillary column. The collected liquid product was adsorbed with H 2 O and analyzed offline by a chromatographic column of HP-5. The turnover frequency (TOF) was calculated based on the total moles of C 3 H 7 CHO divided by the total moles of Rh per hour.
DFT calculation
In the calculation, serials of Rh(PPh 3 ) 2 and HRhCO(PPh 3 ) 2 models are constructed. Distinguished from homogeneous catalysts, PPh 3 , as a ligand, was subjected to specific structural constraints during the computational analysis. The three farthest protons of the three benzene rings opposite to the P atom are immobilized to restrict the mobility of coordinated P atoms. The distances between the three hydrogen atoms are fixed at 7.7 angstroms. All computations were conducted utilizing the Gaussian 16 software package 52 . Density functional theory (DFT) 53 , 54 , 55 was applied using the B3LYP hybrid exchange-correlation functional 56 , 57 , 58 . To account for relativistic effects consistently, a mixed basis set was employed for geometry optimizations, comprising the effective core potential LanL2DZ basis set for the Rhodium (Rh) atom and the 6–31 G+ (d, p) basis set for non-metal atoms (C, H, O, and P) 59 , 60 , 61 . The calculation of total energy also incorporated the consideration of zero-point energy.
Data availability
All data supporting the findings of this study are available within the paper, and its Supplementary Information files. The source data are available from the corresponding authors on request. Source data are provided with this paper.
Beller, M., Cornils, B., Frohning, C. D. & Kohlpaintner, C. W. Progress in hydroformylation and carbonylation. J. Mol. Catal. A: Chem. 104 , 17–85 (1995).
Article CAS Google Scholar
Franke, R., Selent, D. & Börner, A. Applied hydroformylation. Chem. Rev. 112 , 5675–5732 (2012).
Article CAS PubMed Google Scholar
Wu, X.-F., Han, B., Ding, K. & Liu, Z. The Chemical Transformations of C1 Compounds (John Wiley & Sons, 2022).
Cole-Hamilton, D. Homogeneous catalysis-new approaches to catalyst separation, recovery, and recycling. Science 299 , 1702–1706 (2003).
Article ADS CAS PubMed Google Scholar
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeO x. Nat. Chem. 3 , 634–641 (2011).
Copéret, C., Chabanas, M., Petroff Saint‐Arroman, R. & Basset, J. M. Homogeneous and heterogeneous catalysis: bridging the gap through surface organometallic chemistry. Angew. Chem. Int. Ed. 42 , 156–181 (2003).
Article Google Scholar
Cui, X., Li, W., Ryabchuk, P., Junge, K. & Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 1 , 385–397 (2018).
Kaiser, S. K., Chen, Z., Faust Akl, D., Mitchell, S. & Perez-Ramirez, J. Single-atom catalysts across the periodic table. Chem. Rev. 120 , 11703–11809 (2020).
Liu, Y. et al. Rhodium nanoparticles supported on silanol-rich zeolites beyond the homogeneous Wilkinson’s catalyst for hydroformylation of olefins. Nat. Commun. 14 , 2531 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Qi, L. et al. Ethene Hydroformylation catalyzed by rhodium dispersed with zinc or cobalt in silanol nests of dealuminated zeolite beta. J. Am. Chem. Soc. 145 , 2911–2929 (2023).
Wei, B. et al. Single-atom gold species within zeolite for efficient hydroformylation. Chem. Catal. 2 , 2066–2076 (2022).
Shang, W. et al. Efficient heterogeneous hydroformylation over zeolite-encaged isolated rhodium ions. CCS Chem. 5 , 1526–1539 (2023).
Zhao, M. et al. Low-temperature hydroformylation of ethylene by phosphorous stabilized Rh sites in a one-pot synthesized Rh-(O)-P-MFI zeolite. Nat. Commun. 14 , 7174 (2023).
Zheng, Y. et al. Boosting the hydroformylation activity of a Rh/CeO 2 single-atom catalyst by tuning surface deficiencies. ACS Catal. 13 , 7243–7255 (2023).
Li, T. et al. Styrene hydroformylation with in situ hydrogen: regioselectivity control by coupling with the low-temperature water–gas shift reaction. Angew. Chem. Int. Ed. 59 , 7430–7434 (2020).
Ro, I. et al. Bifunctional hydroformylation on heterogeneous Rh-WOx pair site catalysts. Nature 609 , 287–292 (2022).
Lang, R. et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh 3 ) 3 . Angew. Chem. Int. Ed. 55 , 16054–16058 (2016).
Escobar-Bedia, F. J. et al. Active and regioselective Ru single-site heterogeneous catalysts for alpha-olefin hydroformylation. ACS Catal. 12 , 4182–4193 (2022).
Gao, P. et al. Phosphorus coordinated Rh single-atom sites on nanodiamond as highly regioselective catalyst for hydroformylation of olefins. Nat. Commun. 12 , 4698 (2021).
Jiang, M. et al. Ultrastable 3V-PPh 3 polymers supported single Rh sites for fixed-bed hydroformylation of olefins. J. Mol. Catal. A: Chem. 404 , 211–217 (2015).
Li, C. et al. Single atom dispersed Rh-biphephos&PPh 3 @ porous organic copolymers: highly efficient catalysts for continuous fixed-bed hydroformylation of propene. Green. Chem. 18 , 2995–3005 (2016).
Article ADS CAS Google Scholar
Li, C. et al. Xantphos doped Rh/POPs-PPh 3 catalyst for highly selective long-chain olefins hydroformylation: chemical and DFT insights into Rh location and the roles of Xantphos and PPh 3 . J. Catal. 353 , 123–132 (2017).
Ji, G. et al. The effect of the position of cross-linkers on the structure and microenvironment of PPh 3 moiety in porous organic polymers. J. Mater. Chem. A 9 , 9165–9174 (2021).
Feng, S. et al. Sulfur poisoning and self-recovery of single-site Rh1/porous organic polymer catalysts for olefin hydroformylation. Angew. Chem. Int. Ed. 62 , e202304282 (2023).
Liu, B. et al. Heterogeneous hydroformylation of alkenes by Rh-based catalysts. Chem 8 , 2630–2658 (2022).
van Leeuwen, P. W., Kamer, P. C., Reek, J. N. & Dierkes, P. Ligand bite angle effects in metal-catalyzed C−C bond formation. Chem. Rev. 100 , 2741–2770 (2000).
Article PubMed Google Scholar
Freixa, Z. & Van Leeuwen, P. W. Bite angle effects in diphosphine metal catalysts: steric or electronic? Dalton Trans. 10 , 1890–1901 (2003).
Casey, C. P. & Whiteker, G. T. The natural bite angle of chelating diphosphines. Isr. J. Chem. 30 , 299–304 (1990).
van der Veen, L. A. et al. Electronic effect on rhodium diphosphine catalyzed hydroformylation: the bite angle effect reconsidered. J. Am. Chem. Soc. 120 , 11616–11626 (1998).
Jeffrey, J. C. & Rauchfuss, T. B. Metal complexes of hemilabile ligands. Reactivity and structure of dichlorobis (o-(diphenylphosphino) anisole) ruthenium (II). Inorg. Chem. 18 , 2658–2666 (1979).
Bader, A. & Lindner, E. Coordination chemistry and catalysis with hemilabile oxygen-phosphorus ligands. Coord. Chem. Rev. 108 , 27–110 (1991).
Braunstein, P. & Naud, F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline‐based systems. Angew. Chem. Int. Ed. 40 , 680–699 (2001).
3.0.CO;2-0" data-track-item_id="10.1002/1521-3773(20010216)40:4 3.0.CO;2-0" data-track-value="article reference" data-track-action="article reference" href="https://doi.org/10.1002%2F1521-3773%2820010216%2940%3A4%3C680%3A%3AAID-ANIE6800%3E3.0.CO%3B2-0" aria-label="Article reference 32" data-doi="10.1002/1521-3773(20010216)40:4 3.0.CO;2-0">Article CAS Google Scholar
Adams, G. M. & Weller, A. S. POP-type ligands: variable coordination and hemilabile behaviour. Coord. Chem. Rev. 355 , 150–172 (2018).
Chen, Z., Liu, Z. & Xu, X. Dynamic evolution of the active center driven by hemilabile coordination in Cu/CeO 2 single-atom catalyst. Nat. Commun. 14 , 2512 (2023).
Pelavin, M., Hendrickson, D., Hollander, J. & Jolly, W. Phosphorus 2p electron binding energies. Correlation with extended Hueckel charges. J. Phys. Chem. 74 , 1116–1121 (1970).
Sarma, B. B., Maurer, F., Doronkin, D. E. & Grunwaldt, J.-D. Design of single-atom catalysts and tracking their fate using operando and advanced X-ray spectroscopic tools. Chem. Rev. 123 , 379–444 (2022).
Article PubMed PubMed Central Google Scholar
Varshavsky, Y. S. et al. 13C NMR spectrum of crystalline [Rh(Acac)(CO) 2 ]: a contribution to the discussion on [Rh(Acac)(CO) 2 ] molecular structure in the solid state. J. Organomet. Chem. 874 , 70–73 (2018).
Buisman, G. J. H., van der Veen, L. A., Kamer, P. C. J. & van Leeuwen, P. W. N. M. Fluxional processes in asymmetric hydroformylation catalysts [HRhL ⌒ L(CO)2] containing C2-symmetric diphosphite ligands. Organometallics 16 , 5681–5687 (1997).
Bara-Estaún, A. et al. Multi-nuclear, high-pressure, operando FlowNMR spectroscopic study of Rh/PPh 3 -catalysed hydroformylation of 1-hexene. Faraday Discuss. 229 , 422–442 (2021).
Article ADS PubMed Google Scholar
Dvinskikh, S. V., Zimmermann, H., Maliniak, A. & Sandström, D. Measurements of motionally averaged heteronuclear dipolar couplings in MAS NMR using R-type recoupling. J. Magn. Reson. 168 , 194–201 (2004).
Wu, G. & Wasylishen, R. E. Applications of two-dimensional phosphorus-31 CP/MAS NMR techniques for studying metal phosphine complexes in the solid state. Organometallics 11 , 3242–3248 (1992).
Wu, G. & Wasylishen, R. E. Homonuclear phosphorus-31 J-resolved 2D spectra of rhodium (I) phosphine complexes in the solid state. Inorg. Chem. 31 , 145–148 (1992).
Varshavsky, Y. S., Cherkasova, T., Buzina, N. & Bresler, L. Spectral characteristics of products formed by reaction between Rhacac(PPh 3 )(CO) and methyl iodide. J. Organomet. Chem. 464 , 239–245 (1994).
Gerritsen, L., Van Meerkerk, A., Vreugdenhil, M. & Scholten, J. Hydroformylation with supported liquid phase rhodium catalysts part I. General description of the system, catalyst preparation and characterization. J. Mol. Catal. 9 , 139–155 (1980).
Evans, D., Yagupsky, G. & Wilkinson, G. The reaction of hydridocarbonyltris (triphenylphosphine) rhodium with carbon monoxide, and of the reaction products, hydridodicarbonylbis (triphenylphosphine) rhodium and dimeric species, with hydrogen. J. Chem. Soc. A: Inorg. Phys. Theor. 2660–2665 https://doi.org/10.1039/J19680002660 (1968).
Kamer, P. C., van Rooy, A., Schoemaker, G. C. & van Leeuwen, P. W. In situ mechanistic studies in rhodium catalyzed hydroformylation of alkenes. Coord. Chem. Rev. 248 , 2409–2424 (2004).
Hülsey, M. J. et al. In situ spectroscopy-guided engineering of rhodium single-atom catalysts for CO oxidation. Nat. Commun. 10 , 1330 (2019).
Article ADS PubMed PubMed Central Google Scholar
Spoto, G. et al. IR study of ethene and propene oligomerization on H-ZSM-5: hydrogen-bonded precursor formation, initiation and propagation mechanisms and structure of the entrapped oligomers. J. Chem. Soc. Faraday Trans. 90 , 2827–2835 (1994).
Lashchinskaya, Z. N., Gabrienko, A. A., Prosvirin, I. P., Toktarev, A. V. & Stepanov, A. G. Effect of silver cations on propene aromatization on H-ZSM-5 zeolite investigated by 13 C MAS NMR and FTIR spectroscopy. ACS Catal. 13 , 10248–10260 (2023).
Sivasankar, N. & Frei, H. Direct observation of kinetically competent surface intermediates upon ethylene hydroformylation over Rh/Al 2 O 3 under reaction conditions by time-resolved Fourier transform infrared spectroscopy. J. Phys. Chem. C. 115 , 7545–7553 (2011).
Massiot, D. et al. Modelling one‐and two‐dimensional solid‐state NMR spectra. Magn. Reson. Chem. 40 , 70–76 (2002).
Frisch, M. J. et al. Gaussian 16, Revsion C.01 (Gaussian, Inc., Wallingford, CT, 2016).
Sosa, C. & Lee, C. Density functional description of transition structures using nonlocal corrections. Silylene insertion reactions into the hydrogen molecule. J. Chem. Phys. 98 , 8004–8011 (1993).
Becke, A. D. Density‐functional thermochemistry. II. The effect of the Perdew–Wang generalized‐gradient correlation correction. J. Chem. Phys. 97 , 9173–9177 (1992).
Perdew, J. P. & Wang, Y. Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev. B 46 , 12947–12954 (1992).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98 , 5648–5652 (1993).
Devlin, F., Finley, J., Stephens, P. & Frisch, M. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields: a comparison of local, nonlocal, and hybrid density functionals. J. Phys. Chem. 99 , 16883–16902 (1995).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37 , 785 (1988).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72 , 650–654 (1980).
McLean, A. & Chandler, G. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z= 11–18. J. Chem. Phys. 72 , 5639–5648 (1980).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82 , 270–283 (1985).
Download references
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFA1508003), National Natural Science Foundation of China (Nos. 22302192, 22108275), Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (No. GZB20230724), China Postdoctoral Science Foundation (No. 2024T170900), Doctoral Research Start-up Fund of Liaoning Province (No. 2024-BSBA-28), Strategic Priority Research Program of the Chinese Academy of Sciences (Nos. XDA21020900, XDA29050300), Youth Innovation Promotion Association CAS (No. 2021181). The authors thank the SSRF (BL05U and BL14W1) beamline for experimental data collection.
Author information
These authors contributed equally: Benhan Fan, Miao Jiang.
Authors and Affiliations
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, P.R. China
Benhan Fan, Miao Jiang, Guoqing Wang, Yang Zhao, Lei Ma, Cunyao Li, Li Yan & Yunjie Ding
Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, P.R. China
Bingbao Mei
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, P.R. China
Jingfeng Han
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, P.R. China
Guangjin Hou & Yunjie Ding
School of Chemical Engineering, Dalian University of Technology, Dalian, P.R. China
You can also search for this author in PubMed Google Scholar
Contributions
B.F. and M.J. performed the experiments under the supervision of L.Y. and Y.D. who conceptualized the research and acquired research funding for the project. G.W. helped with the XAFS data analysis. Y.Z. carried out HADDF-STEM imaging. B.M. performed XAFS experiments. J.H. carried out in situ XAFS experiments. L.M. and C.L. helped with data analysis. G.H. helped with NMR sequences and data analysis. T.W. performed DFT calculations. B.F. and M.J. wrote the paper with substantial input and revision from T.W., L.Y. and Y.D. All authors participated in the analysis of experimental data and discussion of the results.
Corresponding authors
Correspondence to Tao Wu , Li Yan or Yunjie Ding .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks PATRICIA Concepcion and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fan, B., Jiang, M., Wang, G. et al. Elucidation of hemilabile-coordination-induced tunable regioselectivity in single-site Rh-catalyzed heterogeneous hydroformylation. Nat Commun 15 , 6967 (2024). https://doi.org/10.1038/s41467-024-51281-1
Download citation
Received : 17 March 2024
Accepted : 31 July 2024
Published : 14 August 2024
DOI : https://doi.org/10.1038/s41467-024-51281-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Physical Review C
Covering nuclear physics.
- Collections
- Editorial Team
Extraction of Gamow-Teller strengths in the β + direction with the ( d , He 2 ) reaction in inverse kinematics
Z. rahman, s. giraud, j. c. zamora, r. g. t. zegers, y. ayyad, s. beceiro-novo, d. bazin, b. a. brown, a. carls, j. chen, m. cortesi, m. denudt, c. maher, w. mittig, f. ndayisabye, s. noji, j. pereira, j. schmitt, m. z. serikow, l. j. sun, j. surbrook, n. watwood, and t. wheeler, phys. rev. c 110 , 024313 – published 14 august 2024.
- No Citing Articles
- INTRODUCTION
- EXPERIMENTAL DETAILS
- DATA ANALYSIS
- RESULTS AND DISCUSSION
- SUMMARY AND OUTLOOK
- ACKNOWLEDGMENTS
The ( d , He 2 ) reaction in inverse kinematics has been developed for experiments with rare-isotope beams to constrain electron-capture rates needed for astrophysical simulations of processes in dense nuclear environments such as supernovae and neutron star crusts. The first experiment focused on the measurement of the O 14 ( d , He 2 ) and N 13 ( d , He 2 ) reactions in inverse kinematics, utilizing the active-target time-projection chamber placed in front of the S800 magnetic spectrograph. This work focuses on the experimental and analysis details, and presents the results for the N 13 ( d , He 2 ) reaction, which is important for constraining electron captures rates on N 13 in the preexplosion convective phase of Type Ia supernova. The extracted Gamow-Teller transition strengths associated with electron capture on N 13 are consistent with those previously obtained from the analog transitions from C 13 . The successful development of the ( d , He 2 ) reaction in inverse kinematics presents a novel opportunity for performing experiments aimed at constraining electron-capture rates in nuclei far from stability.
- Received 19 June 2024
- Accepted 30 July 2024
DOI: https://doi.org/10.1103/PhysRevC.110.024313
©2024 American Physical Society
Physics Subject Headings (PhySH)
- Research Areas
Authors & Affiliations
- 1 Facility for Rare Isotope Beams , Michigan State University , East Lansing, Michigan 48824, USA
- 2 Joint Institute for Nuclear Astrophysics : Center for the Evolution of the Elements, Michigan State University , East Lansing, Michigan 48824, USA
- 3 Department of Physics and Astronomy, Michigan State University , East Lansing, Michigan 48824, USA
- 4 Universidade da Coruña , Campus Industrial de Ferrol, CITENI, Departamento de Física, Ferrol 15403, Spain
- 5 Physics Division, Argonne National Laboratory , Lemont, Illinois 60439, USA
- * Contact author: [email protected]
- † Contact author: [email protected]
- ‡ Contact author: [email protected]
Article Text (Subscription Required)
References (subscription required).
Vol. 110, Iss. 2 — August 2024
Access Options
- Buy Article »
- Log in with individual APS Journal Account »
- Log in with a username/password provided by your institution »
- Get access through a U.S. public or high school library »
Article part of CHORUS

Authorization Required
Other options.
- Buy Article »
- Find an Institution with the Article »
Download & Share
ToF (in ns) between the two scintillators placed at the exit of A1900 separator and the entrance of the beam line to the S800 spectrograph, illustrating the event-by-event particle identification of the incoming beam particles. The data shown are from the runs for which the S800 spectrograph was set at a magnetic rigidity of B ρ = 3.0582 Tm.
(a) B ρ ranges of the CE reaction products or their decay products produced in the ( d , He 2 ) reaction on the O 14 , N 13 , and C 12 isotopes in the cocktail beam. For each of the produced isotopes, the horizontal bars indicate the full width of the B ρ distribution, taking into consideration the momentum kicks induced through the decay by particle emission at the highest excitation energy at which that particle was or could be observed. The bottom row indicates the three B ρ settings used in the experiment. In this case, the horizontal bars and green bands indicate the B ρ acceptance ( ± 3 % ) for each setting. (b) Measured B ρ distributions of N 14 and C 12 for the O 14 ( d , He 2 ) reaction, taken at the central B ρ setting of 3.0582 Tm.
Particle identification with the S800 spectrograph after gating on events in which the incoming beam particles are identified as N 13 .
(a) The drift length distribution for 30 runs before (in blue) and after (in red) drift-velocity correction. (b) Drift-length distributions for two runs before drift-velocity correction, indicating that the correction is run dependent. For comparison, the distributions for both runs are normalized to 1. (c) The maximum drift length all as a function of run number before (red) and after (blue) drift velocity correction. The plotted uncertainties represent the uncertainties in the fit of the maximum drift distance.
(a) Example of a ( d , He 2 ) event in the AT-TPC together with the fitted lines following the RANSAC algorithm. (b) Determination of the closest distance between the two tracks, the vertex location, and the last points of each track for the same events as shown in (a). (c) The same event as in (a) and (b) but from a different perspective.
Correlations between the relative energy ε p p and the center-of-mass scattering angle θ c.m. for the O 14 ( d , He 2 ) N 14 ( 1 + ; 3.95 MeV) reaction inside the sensitive region of AT-TPC as a function of scattering angle for the O 14 ( d , He 2 ) N 14 reaction in (a) the simulation and (b) the data.
Simulated fragment acceptance as a function of excitation energy in C 13 for events obtained by gating on C 12 particle.
Differential cross section for the ( a ) N 13 ( d , He 2 ) and (b) O 14 ( d , He 2 ) reactions for θ c.m. ≤ 8 ∘ . The dashed lines represent separation energies for different decay channels and the different colors indicate which residual particle was detected in the S800 spectrograph, as labeled in the figure. (a) shows differential cross section up to 22 MeV for the N 13 ( d , He 2 ) reaction, but may have missing cross section above 17.5 MeV, which is the threshold for the decay by proton emission, as the B 13 fragment was not detected in the S800 spectrograph focal plane for the selected B ρ settings.
Calculated differential cross section as a function of center-of-mass scattering angle for Δ L = 0 , 1, and 2 components for the N 13 ( d , He 2 ) reaction at an excitation energy of 3.68 MeV in C 13 . (a) shows the differential cross sections obtained directly from the accba code. (b) shows the differential cross section obtained after accounting for the ε p p acceptance by using the attpcroot simulation code. Note that in this plot, the error bars are indicative of the statistical uncertainties in the Monte Carlo simulations. Also note that the differential cross sections in both plots have been scaled so that their maxima are approximately identical for visualization purposes.
MDA results for different ranges in excitation energy, as discussed in the text.
Comparison of the extracted GT strengths from the N 13 ( d , He 2 ) reaction with shell model calculations using the CKII interaction in the p -shell-model space, and strengths extracted for the analog C 13 ( He 3 , t ) reaction, calibrated with β -decay data [ 31, 40 ].
Sign up to receive regular email alerts from Physical Review C
- Forgot your username/password?
- Create an account
Article Lookup
Paste a citation or doi, enter a citation.

IMAGES
COMMENTS
How it works. Reaction time is the time between any kind of event and the response it elicits in a system. The brain is an essential part of developing a quick reaction time. In this experiment, the eye sees that the ruler has been dropped. This information travels from sensory neurons along the optic nerve from the eye to the brain.
In the simple reaction time task, you need to wait until you see a black cross on the white square. When that happens, you press as soon as you can the space bar. Thus, there is one stimulus (black cross) and one response (pressing the space bar). In the choice reaction time task, you need to wait until you see a black cross on one of the four ...
We can use the distance the meter stick fell before you caught it to figure out your reaction time. The following formula is the basis: d = 1/2 gt 2. In this formula, "d" equals the distance the object fell, "g" equals gravitational acceleration (9.8 m/s 2 ), and "t" is the time the object was falling. To simplify the process, we ...
The equation to calculate averages is: Average = (trial 1 + trial 2 + trial 3 + trial 4 + trial 5)/5. Use the average distance you calculated in Step 9 and refer to the table below to find the average speed of reaction time for each volunteer. Record this value in the row 'Average Reaction Time' for each column.
Here it is! The average reaction time for humans is 0.25 seconds to a visual stimulus, 0.17 for an audio stimulus, and 0.15 seconds for a touch stimulus. Concise Handout for the Classroom This handout was designed by Virginia Johnson, a graduate student who adapted our experiment here to use as a teaching tool. This handout provides great ...
Calculate reaction time in seconds as before. A computer-based test of reaction times appear below. Go to the web site and follow the instructions. Method 4: This is a 'choice reaction time test' which tests how fast you can respond to the random appearance of dots in a grid over the course of 30 seconds.
In this experiment, you will measure your reaction time by catching a metric ruler with your fingers. After you catch the ruler, you will convert your measurement in centimeters into a reaction time measured in seconds. To do this, you will need to use the following reaction time table (from Brody, 1987, 147):
On average, reaction time takes between 150 and 300 milliseconds. If that sounds like a long time, think about how much has to happen for you to react. When your eye sees the ruler falling ...
Reaction Time Experiment. We can test the time it takes for our bodies to react to stimuli with this simple reaction time experiment. I've prepared a printable download to help record and analyze your data. (download instructions at the end of the post) Supplies. meter stick or ruler. a partner. chair.
Reaction time has been used to measure age-related response quality 2). There are three types of reaction time tasks: simple reaction time (simple RT), choice reaction time ... To this end, we conducted two experiments. The first consisted of simple RT and choice RT tasks; the second consisted of a go/no-go RT task in which responses were ...
The neural pathway involved in a reaction time experiment involves a series of neural processes. This experiment does not test a simple reflex. Rather, this activity is designed to measure the response time to something that you see. Catching a dropped ruler begins with the eye watching the ruler in anticipation of it falling.
1 Experimental Study of Human Reaction Time Experimental Study of Human Reaction Time. This lab is designed to align with AAOT science outcome #1: Gather, comprehend, and communicate scientific and technical information in order to explore ideas, models, and solutions and generate further questions. Materials: 12″ (30 cm) Ruler; digital device with spreadsheet program
interpreting, and evaluating reaction-time (RT) experiments. Theseissues are best considered in relation to particular substantive questions and interpretations, but time limitations prevent this. ... then their effects on mean reaction time should be additive.That is, the effect of (changing the levelof) F on mean RTshould be invariant as the ...
How to test reaction time with a ruler. You can test reaction times using just a ruler. Simple ruler drop reaction time test What you need. 30cm ruler. Pen and Paper. Volunteers. How to test your reaction time. Hold the top of the ruler with your arm stretched out. Your fingers should be on the highest measurement.
This is an experiment that demonstrates how to determine your reaction time by catching a falling meter ruler.
Reaction time is defined to be the amount of time between the occurrence of an event (such as the car pulling out into the road) and a person's response (hitting the brakes). Researchers long ago discovered that complicated decisions lead to slower reaction times. By carefully manipulating tasks, we can identify the different throught processes ...
Reaction time in psychology research is used to quantify cognitive processes and behaviors. A clear-cut definition of reaction time has to do with the amount of time passed between an appeared stimulus and the response. There are two components to measuring reaction time, the stimulus' time of onset and when the participant's response ...
In this science fair project, you won't be looking at memory tests, but at reaction time tests, and investigating whether eating peppermint can improve reaction times when a person is tired, or under mental fatigue. Reaction time is the time between the start of a sensory stimulus and the time when a person responds to that stimulus.
Mr Wakeford shows you how to test your reaction time for GCSE Biology.
Maria and Katie from St Mary's conducted an experiment, in which they found that the average reaction time for their left hand was 0.2s, while for their right hand it was 0.15s. Rosie, Natalie and Gabby, also from St Mary's provided similar data which supports the argument that we react quicker with our better hand.
The average score of this reaction time test is 273 ms. A lower number means your reaction to the on-screen prompt took less time to click. A higher score means you were slower to react and click. So, if you score lower than 273 ms, you are already in a good place. However, if you scored a higher number, you will need to practice more and hone ...
N/a reaction time lab report introduction: reaction time is the time between the presentation of stimulus and the initiation of the muscular response to that ... Several different factors affect reaction time. This experiment was performed in order to measure the time it took an individual to react in response to a visual stimulus, as well as ...
Find out in the emergency stop game. Powered by JustPark. In a moment you'll start driving. When you see the sign click or press any key to stop. sign tap the screen to stop. We'll guess your age based on your reactions. Do you have the reflexes of an 18 year old? Take this simple test, and we'll gauge your age based on your reaction time.
Rachael Gunn, also known as B-Girl Raygun, spoke out Thursday after several whirlwind days of memes, accusations and conspiracy theories surrounding her Olympic Games performance.
Aussie breaker Rachael Gunn, known as B-girl Raygun, took the internet by storm after her brief but memorable time on stage at the Paris 2024 Olympics. In head-to-head battles against b-girls from ...
After the NMR experiments, the Rh-POPs-active sample was treated on a vacuum line at room temperature and purged with 0.17 MPa C 3 H 6, then raised the temperature to 363 K and reacted for 30 ...
The (d, He 2) reaction in inverse kinematics has been developed for experiments with rare-isotope beams to constrain electron-capture rates needed for astrophysical simulations of processes in dense nuclear environments such as supernovae and neutron star crusts.The first experiment focused on the measurement of the O 14 (d, He 2) and N 13 (d, He 2) reactions in inverse kinematics, utilizing ...