- Structure of Atom
- Oil Drop Experiment

Milliken's Oil Drop Experiment
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community.
Millikens Oil Drop Experiment Definition
In the experiment, Milliken allowed charged tiny oil droplets to pass through a hole into an electric field. By varying the strength of the electric field the charge over an oil droplet was calculated, which always came as an integral value of ‘e.’
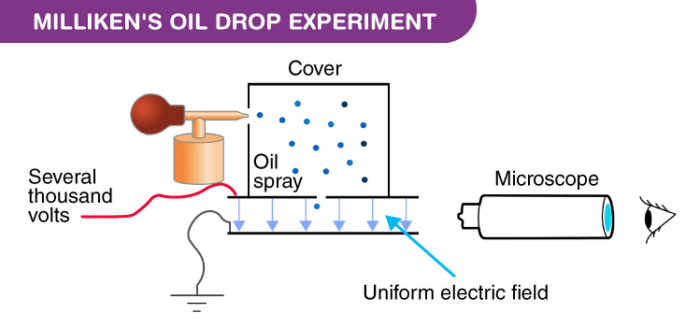
Apparatus of the Milliken’s Oil Drop Experiment
The apparatus for the experiment was constructed by Milliken and Fletcher. It incorporated two metal plates held at a distance by an insulated rod. There were four holes in the plate, out of which three were there to allow light to pass through them and one was there to allow viewing through the microscope.
Ordinary oil wasn’t used for the experiment as it would evaporate by the heat of the light and so could cause an error in the Millikens Oil Drop Experiment. So, the oil that is generally used in a vacuum apparatus which is of low vapour pressure was used.
Milliken’s Oil Drop Experiment Procedure
- Oil is passed through the atomizer from where it came in the form of tiny droplets. They pass the droplets through the holes present in the upper plate of the apparatus.
- The downward motions of droplets are observed through a microscope and the mass of oil droplets, then measure their terminal velocity.
- The air inside the chamber is ionized by passing a beam of X-rays through it. The electrical charge on these oil droplets is acquired by collisions with gaseous ions produced by ionization of air.
- The electric field is set up between the two plates and so the motion of charged oil droplets can be affected by the electric field.
- Gravity attracts the oil in a downward direction and the electric field pushes the charge upward. The strength of the electric field is regulated so that the oil droplet reaches an equilibrium position with gravity.
- The charge over the droplet is calculated at equilibrium, which is dependent on the strength of the electric field and mass of droplet.
Milliken’s Oil Drop Experiment Calculation
F up = F down
F up = Q . E
F down = m.g
Q is an electron’s charge, E is the electric field, m is the droplet’s mass, and g is gravity.
One can see how an electron charge is measured by Millikan. Millikan found that all drops had charges that were 1.6x 10 -19 C multiples.
Milliken’s Oil Drop Experiment Conclusion
The charge over any oil droplet is always an integral value of e (1.6 x 10 -19 ). Hence, the conclusion of Millikens Oil Drop Experiment is that the charge is said to be quantized, i.e. the charge on any particle will always be an integral multiple of e.
Frequently Asked Questions – FAQs
What did millikan’s oil drop experiment measure.
Millikan oil-drop test, the first simple and persuasive electrical charge calculation of a single electron. It was first conducted by the American physicist Robert A. in 1909. He discovered that all the drops had charges that were simple multiples of a single integer, the electron’s fundamental charge.
What is the importance of Millikan’s oil drop experiment?
The experiment with Millikan is important since it defined the charge on an electron. Millikan used a very basic, very simple system in which the behaviour of gravitational, electrical, and (air) drag forces were controlled.
What did Millikan conclude after performing his oil drop experiment?
An integral multiple of the charge on an electron is the charge on every oil decrease. About an electric force. In a relatively small amount, the charge and mass of the atom must be condensed.
Why charges are quantized?
Charges are quantized since every object’s charge (ion, atom, etc.) Charge quantization, therefore, implies that no random values can be taken from the charge, but only values that are integral multiples of the fundamental charge (proton / electron charge).
Can charge be created or destroyed?
The Charge Conservation Law does not suggest that it is difficult to generate or remove electrical charges. It also means that any time a negative electrical charge is produced, it is important to produce an equal amount of positive electrical charge at the same time so that a system’s overall charge does not shift.
For more information about quantum physics , download BYJU’S-The learning app to play store and app store.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Why does physics work in SI units?

Millikan oil-drop experiment
Our editors will review what you’ve submitted and determine whether to revise the article.
- PhysicsLAB - Millikan's Oil Drop Experiment

Millikan oil-drop experiment , first direct and compelling measurement of the electric charge of a single electron . It was performed originally in 1909 by the American physicist Robert A. Millikan , who devised a straightforward method of measuring the minute electric charge that is present on many of the droplets in an oil mist. The force on any electric charge in an electric field is equal to the product of the charge and the electric field. Millikan was able to measure both the amount of electric force and magnitude of electric field on the tiny charge of an isolated oil droplet and from the data determine the magnitude of the charge itself.
Millikan’s original experiment or any modified version, such as the following, is called the oil-drop experiment. A closed chamber with transparent sides is fitted with two parallel metal plates, which acquire a positive or negative charge when an electric current is applied. At the start of the experiment, an atomizer sprays a fine mist of oil droplets into the upper portion of the chamber. Under the influence of gravity and air resistance, some of the oil droplets fall through a small hole cut in the top metal plate. When the space between the metal plates is ionized by radiation (e.g., X-rays ), electrons from the air attach themselves to the falling oil droplets, causing them to acquire a negative charge. A light source, set at right angles to a viewing microscope , illuminates the oil droplets and makes them appear as bright stars while they fall. The mass of a single charged droplet can be calculated by observing how fast it falls. By adjusting the potential difference, or voltage, between the metal plates, the speed of the droplet’s motion can be increased or decreased; when the amount of upward electric force equals the known downward gravitational force, the charged droplet remains stationary. The amount of voltage needed to suspend a droplet is used along with its mass to determine the overall electric charge on the droplet. Through repeated application of this method, the values of the electric charge on individual oil drops are always whole-number multiples of a lowest value—that value being the elementary electric charge itself (about 1.602 × 10 −19 coulomb). From the time of Millikan’s original experiment, this method offered convincing proof that electric charge exists in basic natural units. All subsequent distinct methods of measuring the basic unit of electric charge point to its having the same fundamental value.
The Millikan Oil Drop Experiment
Theresa Knott / Wikimedia Commons / CC BY-SA 3.0
- Physical Chemistry
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Robert Millikan's oil drop experiment measured the charge of the electron . The experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. The choice of oil was important because most oils would evaporate under the heat of the light source, causing the drop to change mass throughout the experiment. Oil for vacuum applications was a good choice because it had a very low vapor pressure. Oil droplets could become electrically charged through friction as they were sprayed through the nozzle or they could be charged by exposing them to ionizing radiation . Charged droplets would enter the space between the parallel plates. Controlling the electric potential across the plates would cause the droplets to rise or fall.
Calculations for the Experiment
F d = 6πrηv 1
where r is the drop radius, η is the viscosity of air and v 1 is the terminal velocity of the drop.
The weight W of the oil drop is the volume V multiplied by the density ρ and the acceleration due to gravity g.
The apparent weight of the drop in air is the true weight minus the upthrust (equal to the weight of air displaced by the oil drop). If the drop is assumed to be perfectly spherical then the apparent weight can be calculated:
W = 4/3 πr 3 g (ρ - ρ air )
The drop is not accelerating at terminal velocity so the total force acting on it must be zero such that F = W. Under this condition:
r 2 = 9ηv 1 / 2g(ρ - ρ air )
r is calculated so W can be solved. When the voltage is turned on the electric force on the drop is:
F E = qE
where q is the charge on the oil drop and E is the electric potential across the plates. For parallel plates:
E = V/d
where V is the voltage and d is the distance between the plates.
The charge on the drop is determined by increasing the voltage slightly so that the oil drop rises with velocity v 2 :
qE - W = 6πrηv 2
qE - W = Wv 2 /v 1
- Organic Chemistry Introduction
- Standard Molar Entropy Definition in Chemistry
- What Is a Molotov Cocktail? Definition and Explanation
- Force Definition and Examples (Science)
- Exothermic Reaction Examples - Demonstrations to Try
- What Is Valence or Valency?
- How to Make a Roman Candle Firework
- Quantum Numbers and Electron Orbitals
- Black Powder Composition
- The Octet Rule Explanation in Chemistry
- The Chemistry Behind Sparklers
- The Chemistry of Firework Colors
- Chemical Elements in Fireworks
- The Discarded Phlogiston Theory in Early Chemistry History
- Subatomic Particles You Should Know
- Valency of Elements on the Periodic Table

The Millikan Oil Drop Experiment

Introduction To The Millikan Oil Drop Experiment
In this article, you will learn all you need to know (and more) about the Millikan Oil Drop Experiment. If you like this article, check out our other articles!
- Discovering the Nucleus: Rutherford’s Gold Foil Experiment
- Discovering the Electron: JJ Thomson and the Cathode Ray Tube
- Lise Meitner: A Physicist & Chemist Whose Legacy Lives On
- Discovering Nitrogen: Rutherford’s Jar Experiment
Who was Robert A. Millikan?
Robert A. Millikan was born on the 22nd of March, 1868 in Illinois, (U.S.A.). Growing up, Millikan spent most of his childhood living in a rural town called Morrison. Then, in 1875, his family relocated to Maquoketa, Iowa where Millikan started attending Maquoketa high school. Millikan excelled in his learning and decided to further his studies by attending Oberlin College in Ohio. During this time, Millikan started teaching a physics class and decided to pursue the subject as a career. He later obtained his Ph.D. in physics from Columbia University.

After graduating from Columbia, Millikan traveled to the universities of Berlin and Göttingen. There, he furthered his knowledge within his field before returning to the United States to be an assistant at Chicago University’s Ryerson Laboratory. During his time there, Millikan authored (and co-authored) several physics textbooks. Eventually, in 1907, a research project of Millikan’s led to the development of the Oil Drop Experiment .
The Experiment
Devised by Robert A. Millikan and Harvey Fletcher, the Millikan Oil Drop Experiment is conducted in a chamber and is a method of measuring the electric charge of a single electron .
To elaborate, this chamber contains an atomizer, a microscope, a light source, and two parallel metal plates. These metal plates obtain a negative and a positive charge when an electric current would pass through them.

The Procedure
First, the atomizer was to release a fine mist of oil that would drift within the chamber. While drifting, the droplets of oil would make their way into the bottom half of the chamber (between the metal plates) due to a gravitational pull. Here, the oil droplets would be ionized into being negatively charged. Thereafter, while these negatively charged droplets are being pulled down by gravity, the external power-dial would be used to add a charge to the two metal plates (above and below the droplets). Specifically speaking, the top plate would cultivate a positive charge, and a negative charge would be cultivated on the bottom plate.

This creates a situation in which the oppositely charged (positive) metal plate is pulling the negatively charged droplet upwards , while gravity is pulling the droplet downwards . Or in other words, the electrostatic and gravitational forces are now controlling the direction in which the droplet is flowing. Now, if the electrostatic force is greater, then the droplet would rise towards the positively charged plate. Likewise, if the gravitational force is greater than the electrostatic force, then the droplet would be pulled down.
Observations and Conclusion
The purpose of this experiment was to balance these two electrostatic and gravitational forces – which would cause the droplets to halt midair. By doing this, the droplet’s mass, gravitational force, and electrostatic force could be measured, revealing the charge of the electron. Furthermore, by doing these final calculations, Millikan was able to reveal that the charge of an electron would be multiples of 1.602×10−19 Coulombs .
Further Reading
School Chemistry Projects (on ScienceBuddies)
Millikan’s Oil Drop Experiment ( AQA A Level Physics )
Revision note.

Millikan's Oil Drop Experiment
- This experiment was conducted by Millikan and Fletcher in 1909
- It determined the value of fundamental or e = 1.60 × 10 -19 C An electron has a charge - e and a proton has a charge + e " data-title="Elementary Charge (e)" data-toggle="popover">elementary charge

Method for Millikan's Oil Drop Experiment
- Oil is used instead of water because it does not evaporate quickly
- This means the mass of the drops will remain constant
- This consequently changes their charge from neutral
- They will become positively charged if they lose electrons
- They will become negatively charged if they gain electrons
- The drops pass into a region between two metal plates and are viewed using a microscope
Equipment Set Up for Millikan's Oil Drop Experiment

In Millikan's Oil Drop Experiment oil is sprayed into a chamber before passing between metal plates where the electric and gravitational forces are compared
Condition for Stationary Oil Drops
- Negative oil drops with magnitude of charge Q experience an upward force from the uniform electric field
- The magnitude of this force F is:
- For this to occur, the force F has to be equal to the weight of the oil drop, mg , so there is no resultant vertical force on each drop
- Therefore, the condition under which oil drops are held stationary is:
- For that, Milikan needed to determine the mass of each oil drop, so he used Stokes' Law
Worked example
One particular oil drop had a mass of 5.1 × 10 -15 kg. It is held stationary between two charged plates. These are separated by 12 mm and there is a potential difference of 1250 V across them.
Calculate the charge of the oil drop.
Step 1: List the known quantities:
- Mass, m = 5.1 × 10 -15 kg
- Separation of plates, d = 12 mm
- Potential difference, V = 1250 V
- Acceleration due to gravity, g = 9.81 m s -2
Step 2: Recall the condition for a stationary oil drop:
- The condition for the oil drop not to fall or rise:
Step 3: Rearrange this equation to calculate charge:
- Make charge the subject:
The condition for a stationary oil droplet is given in the equation sheet. Focus your revision on using it and understanding where it comes from, as opposed to memorising the equation.
You've read 0 of your 0 free revision notes
Get unlimited access.
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Exams
the (exam) results speak for themselves:
Did this page help you?
Author: Dan MG
Dan graduated with a First-class Masters degree in Physics at Durham University, specialising in cell membrane biophysics. After being awarded an Institute of Physics Teacher Training Scholarship, Dan taught physics in secondary schools in the North of England before moving to SME. Here, he carries on his passion for writing enjoyable physics questions and helping young people to love physics.
Description This simulation is a simplified version of an experiment done by Robert Milliken in the early 1900s. Hoping to learn more about charge, Milliken sprayed slightly ionized oil droplets into an electric field and made observations of the droplets. When the voltage is zero and the run button is pressed, the drop will fall due to the force of gravity. It will reach a terminal velocity (v t ) as it falls. Pause the simulation while you record the terminal velocity. This terminal velocity can be used to determine the mass of the drop. Use the equation: mass = kv t 2 to determine the mass of the particle. The value of k in this simulation is 4.086 x 10 -17 kg s 2 /m 2 . Once the terminal velocity is recorded and the mass calculated, with the simulation still paused increase the voltage between the plates until the two force vectors are approximately equal length. This will produce an upward field and an upward force on the positive droplets. If the upward force of the electric field is equal to the downward force of gravity, and the drag force is zero, the particle will not accelerate. To be sure that the lack of acceleration is not related to drag forces, the velocity must also be zero as well as the acceleration in order to be sure that the two forces are balanced. Increase and decrease the voltage (use the left/right arrow keys) until both the acceleration and velocity are at zero. The velocity may not stay at exactly zero, but find the voltage that has the velocity changing most slowly as it passes v = 0. Use the methods discussed above to ultimately determine the charge on ten (or more) different oil-drops. Use V = Ed to calculate the field strength (d = 5 cm = 0.05 m). Use Eq = mg when the velocity is zero to determine the charge q on the droplet. Record all your data in a table or spreadsheet. After you get each q, create a new particle and start again. When you have the table filled in, look at the various values for q. Is there any pattern to them, or are they seemingly random? Can you draw any conclusions from the Q measurements?

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Who Did the Oil Drop Experiment?
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron . Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general.
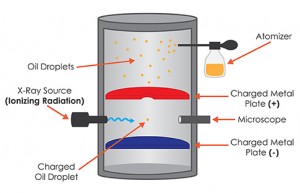
What is the Oil Drop Experiment?
In the original version, Millikan and one of his graduate students, Harvey Fletcher, took a pair of parallel horizontal metallic plates. A uniform electric field was created in the intermediate space by applying a potential difference between them. The plates were held apart by a ring of insulating material. The ring had four holes, three for allowing light to illuminate the setup, and the fourth one enabled a microscope for viewing. A closed chamber with transparent walls was fitted above the plates.
At the beginning of the experiment, a fine mist of oil droplets was sprayed into the chamber. In modern setups, an atomizer replaces the oil droplets. The oil was so chosen such that it had a low vapor pressure and capable of charging. Some of the oil drops became electrically charged by friction as they forced their way out of the nozzle. Alternatively, charging could also be induced by incorporating a source of ionizing radiation , such as an X-Ray tube, in the apparatus. The droplets entered the space between the plates and raised or fell, according to the requirement, by varying plate voltage.
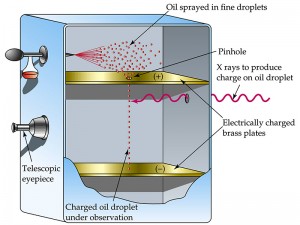
In terms of the present-day arrangement, when the electric field is turned off, the oil drops fall between the plates under the action of gravity only. The friction with the oil molecules in the chamber makes them reach their terminal velocity fast. The terminal velocity is the constant speed that a freely falling object eventually reaches when the resistance of the medium through which it is falling prevents further acceleration . Once the field is turned on, the charged drops start to rise. This motion happens since the electric force directed upwards is stronger than the gravitational force acting downwards. One charged drop is selected and kept at the center of the field of view of the microscope after allowing all other drops to fall by alternately switching off the voltage source. The experiment is conducted with this drop.
Theory and Calculations
First, the oil drop is allowed to fall in the absence of an electric field, and its terminal velocity, say v 1 , is found out. Using Stokes’ law, the drag force acting on the drop is calculated using the following formula.
Here r is the radius of the drop and ɳ, the viscosity of air.
The weight of the drop, w’, which is the product of its mass and acceleration due to gravity g, is given by the equation,
where ρ is the density of the oil.
However, what we need here is the apparent weight w of the drop in the air given by the difference of the actual weight and the upthrust of the air. We can express w by the following formula.
Here ρ air denotes the density of air.
When the drop attains terminal velocity, then it has no acceleration. Hence, the total force acting on it must be zero. That means,
The above equation can be used to find out the value of r. Once r is calculated, the value of w can easily be found out from equation (i) marked above.
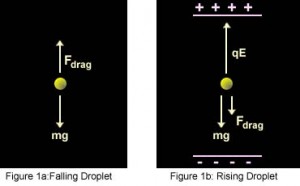
Now after turning on the electric field between the plates, the electric force F E acting on the drop is,
Where E is the electric field and q the charge on the oil drop. For parallel plates, the formula for E is,
Here V is the potential difference and d the distance between the plates. That implies,
Now if we adjust V to make the oil drop remain steady at a point, then
Thus, the value of q can be calculated. By repeatedly applying this method to multiple oil droplets, the electric charge values on individual drops were always found to be integer multiples of the smallest value. This lowest charge could be nothing but the charge on the elementary particle, electron. By this method, the electronic charge was calculated to be approximate, 1.5924×10 −19 C, making an error of 1% of the currently accepted value, 1.602176487×10 −19 C. All subsequent research pointed to the same value of charge on the fundamental particle.
Millikan was able to measure both the amount of electric force and magnitude of electric field on the tiny charge of an isolated oil droplet and from the data determine the magnitude of the charge itself. Millikan’s oil drop experiment proved that the electric charge is quantized in nature. The electric charge appears in quanta of magnitude 1.6 X 10 -19 C in oil droplets.
Robert Millikan’s Oil Drop Experiment Animation
Millikan’s oil drop experiment and the atomic theory.
Until the time of the Oil Drop Experiment, the world had little or no knowledge of what is present inside an atom . Earlier experiments by the English Physicist J.J. Thomson had shown that atoms contain some negatively charged particles of masses significantly smaller than that of the hydrogen atom. Nevertheless, the exact value of the charge carried by these subatomic particles remained in the dark. The very existence of these particles was not accepted by many due to a lack of concrete evidence. Thus, the atomic model was shrouded in mystery. In this scenario, with Millikan’s groundbreaking effort to quantify the charge on an electron, the atomic theory came of age in the early years of the twentieth century.
Controversy about the Oil Drop Experiment and Discovery
Robert Millikan was the sole recipient of the Nobel Prize in Physics in 1923 for both his work in this classic experiment and his research in the photoelectric effect . Fletcher’s work on the oil drop project, however, was not recognized. Many years later, the writings of Fletcher revealed that Millikan wished to take the sole credit for the discovery in exchange for granting him a Ph.D. and helping him secure a job after his graduation.
The beauty of the oil drop experiment lies in its simple and elegant demonstration of the quantization of charge along with measuring the elementary charge on an electron that finds widespread applications to this day. With the progress of time, considerable modifications have been made to the original setup resulting in obvious perfection in the results. Still, no substantial deviation from the results of the classical experiment could yet be found.
- Robert Millikan and Harvey Fletcher conducted the oil drop experiment to determine the charge of an electron. The experiment was the first direct and riveting measurement of the electric charge of a single electron.
- They suspended tiny charged droplets of oil between two metal electrodes by balancing downward gravitational force with upward drag and electric forces.
- They later used their findings to determine the mass of the electron.
- Kentchemistry.com
- Physics.utah.edu
- Nobelprize.org
- Ffden-2.phys.uaf.edu
- Chem.libretexts.org
Article was last reviewed on Thursday, February 2, 2023
Related articles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles
Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Talk to our experts
1800-120-456-456
- Millikan's Oil Drop Experiment

Introduction
The oil drop experiment was performed in 1909 by Robert A. Millikan and Harvey Fletcher to measure the elementary electric charge (it means the electron's charge). This experiment took place in the Ryerson Physical Laboratory, which is present at the University of Chicago. Also, this experiment has proved to be very crucial in physics.
Before this experiment, the existence of subatomic particles was not accepted universally. Millikan's apparatus has an electric field created between a parallel pair of metal plates held apart by an insulating material. The oil droplets, which are electrically charged, enter the electrical field and are balanced between two plates by altering the field. When the charged drops fell at a constant rate, the gravitational forces and electric forces on it were equal.
Principles of Millikan's Experiment
The Millikan experiment is complicated and fiddly while performing in school. It is more likely that we will use a simulation or a film clip of the experiment to show its principles to the students. Few of such principles are,
An oil drop can fall under its own weight. If a charge is given to the drop, it can be suspended by using an electric field. At this point, the electrostatic force balances the weight of every drop. Then the size of the electrostatic force depends entirely on the drop. So Millikan should have figured out the charge as soon as he knew the weight.
Millikan allowed the drop to fall through the air to find the weight of the drop. It reaches its terminal velocity quickly. At this point, the weight is balanced by the viscous drag of the air. Drag can be calculated from the Stokes' Law, which allowed Millikan to determine the weight.
Millikan repeated the same experiment thoroughly for over 150 oil drops and selected 58 of Millikan oil drop experiment results and got to find the highest common factor. It means the single unit of charge that could be multiplied up to give the charge he measured on all of his oil drops.
Oil Drop Experiment
Millikan allowed charged small oil droplets to travel through a hole into an electric field in the experiment. With the electric field's varying strength, the charge over an oil droplet is calculated, and it always comes as a fundamental value of 'e.'
(Image will be uploaded soon)
Millikan and Fletcher designed the experiment apparatus. It included two metal plates held at a distance by an insulated rod. There were four holes in the plate, three of which were there to allow light to pass through, and one was there to allow viewing through the microscope.
They did not use ordinary oil for this experiment, as it would evaporate by the heat of the light, and could cause an error in the Millikan Oil Drop Experiment. The oil, which is usually used in a vacuum apparatus with low vapour pressure, was also used.
Oil passes through the atomizer, from where it came in tiny droplets form. The same droplets pass through the holes in the upper plate of the apparatus.
The droplet's downward movements are observed through the microscope and the mass of the oil droplets, and then their terminal velocity is measured.
The air present inside the chamber is ionised by passing through the X-ray beam. Collisions obtain the electrical charge on these oil droplets with gaseous ions produced by the ionisation of air.
Then, the electric field is set up between the two plates so that the motion of the charged oil droplets can be affected by the same electric field.
Now, gravity attracts the oil in a downward direction, and the electric field pushes the charge upwards. Also, the electric field strength is regulated so that all the oil droplets reach an equilibrium position with gravity.
The charge on the droplet is calculated at equilibrium, which depends on the mass of the droplet and strength of the electric field.
Millikan Oil Drop Experiment Calculations
The experiment initially allows the oil drops to fall between the plates in the absence of the electric field. They accelerate first due to gravity, but gradually the oil droplets slow down because of air resistance.
The Millikan oil drop experiment formula can be given as below.
F up = Q ⋅ E F down = m
Where Q is an electron’s charge, m is the droplet’s mass, E is the electric field, and g is gravity.
Q ⋅ E = m ⋅ g
By this, one can identify how an electron charge is measured by Millikan. Millikan also found that all the drops had charges, which were 1.6x 10 -19 C multiples.
Importance of Millikan's Oil Drop Experiment
Millikan's experiment is quite essential because it establishes the charge on an electron.
Millikan used a simple apparatus in which he balanced the actions of electric, gravitational, and air drag forces.
Using the apparatus, he was able to calculate the charge on an electron as 1.60 × 10 -19 C.
The charge for any oil droplet is always an integral value of e (1.6 x 10 -19 ). Thus, Millikan's Oil Drop Experiment concludes that the charge is said to be quantized, which means that the charge on any particle will be an integral multiple of e always.
Millikan discovered the charge on a single electron using a uniform electric field between the oil drops and two parallel charged plates.

FAQs on Millikan's Oil Drop Experiment
1. What is Millikan’s Oil Drop experiment?
In 1909, Robert Millikan and Harvey Fletcher conducted the canvas drop trial to determine the charge of an electron. They suspended bitsy charged driblets of canvas between two essence electrodes by balancing downcast gravitational force with upward drag and electric forces. The viscosity of the canvas was known, so Millikan and Fletcher could determine the driblets’ millions from their observed diameters (since from the diameters they could calculate the volume and therefore, the mass). Using the known field and therefore the values of graveness and mass, Millikan and Fletcher determined the charge on canvas driblets in mechanical equilibrium. By repeating the trial, they verified that the charges were all multiples of some abecedarian value. They calculated this value to be1.5924 × 10 −19 Coulombs (C), which is within 1 of the presently accepted value of1.602176487 × 10 −19 C. They proposed that this was the charge of one electron.
2. How did the process work?
The outfit incorporated a brace of essence plates and a specific type of canvas. Millikan and Fletcher discovered it had been stylish to use a canvas with a particularly low vapor pressure, similar together designed to be used during a vacuum outfit. Ordinary canvas would dematerialize under the heat of the light source, causing the mass of the canvas to drop to change over the course of the trial.
By applying an implicit difference across a resemblant brace of vertical essence plates, an invariant electric field was created in the space between them. A ring of separating material was used to hold the plates piecemeal. Four holes were dug into the ring — three for illumination by a bright light and another to permit viewing through a microscope. A fine mist of canvas driblets was scattered into a chamber above the plates. The canvas drops came electrically charged through disunion with the snoot as they were scattered. Alternatively, the charge could be convinced by including an ionizing radiation source ( similar to an X-ray tube).
3. Describe the Millikan’s Oil Drop experiment procedure?
Canvas is passed through the atomizer from where it came in the form of bitsy driblets. They pass the driblets through the holes present in the upper plate of the outfit.
The downcast movements of driblets are observed through a microscope and the mass of canvas driblets also measure their terminal haste.
The air inside the chamber is ionised by passing a ray of X-rays through it. The electrical charge on these canvas driblets is acquired by collisions with gassy ions produced by the ionisation of air.
The electric field is set up between the two plates and so the stir of charged canvas driblets can be affected by the electric field.
Graveness attracts the canvas in a downcast direction and the electric field pushes the charge overhead. The strength of the electric field is regulated so that the canvas drop reaches an equilibrium position with graveness.
The charge over the drop is calculated at equilibrium, which depends on the strength of the electrical field and the mass of the drop.
4. Explain Millikan’s Oil Drop experiment in detail?
Millikan’s original trial or any modified interpretation, similar to the following, is called the canvas-drop trial. An unrestricted chamber with transparent sides is fitted with two resemblant essence plates, which acquire a positive or negative charge when an electric current is applied. At the launch of the trial, an atomizer sprays a fine mist of canvas driblets into the upper portion of the chamber. Under the influence of gravity and air resistance, some of the canvas driblets fall through a small hole cut in the top essence plate. When the space between the essence plates is ionized by radiation (e.g., X-rays), electrons from the air attach themselves to the falling canvas driblets, causing them to acquire a negative charge.
A light source, set at right angles to a viewing microscope, illuminates the canvas driblets and makes them appear as bright stars while they fall. The mass of a single charged drop can be calculated by observing how presto it falls. By confirming the implicit difference, or voltage, between the essence plates, the speed of the drop’s stir can be increased or dropped; when the quantum of upward electric force equals the given downcast gravitational force, the charged drop remains stationary. The quantum of voltage demanded to suspend a drop is used along with its mass to determine the overall electric charge on the drop.
Through the repeated operation of this system, the values of the electric charge on individual canvas drops are always whole- number multiples of the smallest value — that value being the abecedarian electric charge itself (about1.602 × 10 −19 coulomb). From the time of Millikan’s original trial, this system offered satisfying evidence that electric charge exists in introductory natural units. All posterior distinct styles of measuring the introductory unit of electric charge point to its having the same abecedarian value.
5. How does Millikan’s Oil Drop experiment work?
Simplified scheme of Millikan’s canvas-drop trial This outfit has a resemblant brace of vertical essence plates. An invariant electric field is created between them. The ring has three holes for illumination and one for viewing through a microscope. A specific type of canvas is scattered into the chamber, where drops come electrically charged. The driblets enter the space between the plates and can be controlled by changing the voltage across the plates.
The driblets entered the space between the plates and, because they were charged, they could be controlled by changing the voltage across the plates. Originally, the canvas drops were allowed to fall between the plates with the electric field turned off. The snappily reached terminal haste due to disunion with the air in the chamber. The field was turned on and, if it was large enough, some of the drops (the charged bones) would start to rise. This is because the overhead electric force, FE, is lesser for them than the down gravitational force,g. (A charged rubber rod can pick up bits of paper in the same way.) A likely-looking drop was named and kept in the middle of the field of view by alternatively switching off the voltage until all the other drops fell. The trial was continued with this single drop. Millikan’s canvas drop trial measured the charge of an electron. Before this trial, the actuality of subatomic patches wasn't widely accepted.
Millikan’s outfit contained an electric field created between a resemblant brace of essence plates, which were held piecemeal by separating material. Electrically charged canvas driblets entered the electric field and were balanced between two plates by altering the field.
6. Why was the Negative Plate Earthed in Millikan's Oil Drop Experiment?
There are three possible reasonable ways to clear it.
The first reason is safety. Grounding ("earthing," in this context), the equipment is so important, particularly the time when you are working with high voltages. The same would be applied to protecting the equipment and for personal safety as well.
The second reason would be to establish a good stable reference point for the voltage measurement. A massive and solidly connected grounding cable would perform that job in a better way.
Finally, from an electrical standpoint, the two plates used in Millikan's experiment form a capacitor. On the other side, this capacitor is being charged to a very high voltage. In such cases, it is suggested to have a discharge path on one of the terminals or plates in order to avoid damage to either humans or equipment as well. Therefore, the negative plate is earthed.
7. Why do we Use Oil Instead of Other Liquids in the Millikan Oil-drop Experiment?
Oil is one of the best liquids for Millikan's oil drop experiment. It retains its mass over a while and exposes to higher temperatures. Also, we employ an atomizer for ultra-fine droplets. So less dense liquids like water and oils are preferred over water because water cannot survive at such higher temperatures.
The atomizer employment is also an important reason behind using oil for this experiment. Moreover, it should be noted that oil would retain the exact volume/mass/weight. This would enable an exact measurement of the charge. Other liquids would separate or dissipate or even evaporate.
August, 1913: Robert Millikan Reports His Oil Drop Results

Robert Millikan’s famous oil drop experiment , reported in August 1913, elegantly measured the fundamental unit of electric charge. The experiment, a great improvement over previous attempts to measure the charge of an electron, has been called one of the most beautiful in physics history, but is also the source of allegations of scientific misconduct on Millikan’s part.
Robert Millikan was born in 1868 and grew up in rural Iowa, the second son of a minister. Millikan attended Oberlin College, earned his PhD from Columbia University, and then spent a year in Germany before taking a position at the University of Chicago.
By about 1906, Millikan had become a successful educator and textbook writer, but he knew that he hadn’t done any research of real scientific significance, and was eager to make his mark as a researcher.
J.J. Thomson had discovered the electron in 1897 and had measured its charge-to-mass ratio. The next step was to determine the electron’s charge separately. Thomson and others tried to measure the fundamental electric charge using clouds of charged water droplets by observing how fast they fell under the influence of gravity and an electric field. The method did give a crude estimate of the electron’s charge.
Millikan saw this opportunity to make a significant contribution by improving upon these measurements. He realized that trying to determine the charge on individual droplets might work better than measuring charge on whole clouds of water. In 1909 he began the experiments, but soon found that droplets of water evaporated too quickly for accurate measurement. He asked his graduate student, Harvey Fletcher, to figure out how to do the experiment using some substance that evaporated more slowly.
Fletcher quickly found that he could use droplets of oil, produced with a simple perfume atomizer. The oil droplets are injected into an air-filled chamber and pick up charge from the ionized air. The drops then fall or rise under the combined influence of gravity, viscosity of the air, and an electric field, which the experimenter can adjust. The experimenter could watch the drops through a specially designed telescope, and time how fast a drop falls or rises. After repeatedly timing the rise and fall of a drop, Millikan could calculate the charge on the drop.
In 1910 Millikan published the first results from these experiments, which clearly showed that charges on the drops were all integer multiples of a fundamental unit of charge. But after the publication of those results, Viennese physicist Felix Ehrenhaft claimed to have conducted a similar experiment, measuring a much smaller value for the elementary charge. Ehrenhaft claimed this supported the idea of the existence of “subelectrons.”
Ehrenhaft’s challenge prompted Millikan to improve on his experiment and collect more data to prove he was right. He published the new, more accurate results in August 1913 in the Physical Review . He stated that the new results had only a 0.2% uncertainty, a great improvement of over his previous results. Millikan’s reported value for the elementary charge, 1.592 x 10 -19 coulombs, is slightly lower than the currently accepted value of 1.602 x 10 -19 C, probably because Millikan used an incorrect value for the viscosity of air.
It appeared that it was a beautiful experiment that had determined quite precisely the fundamental unit of electric charge, and clearly and convincingly established that “subelectrons” did not exist. Millikan won the 1923 Nobel Prize for the work, as well as for his determination of the value of Plank’s constant in 1916.
But later inspection of Millikan’s lab notebooks by historians and scientists has revealed that between February and April 1912, he took data on many more oil drops than he reported in the paper. This is troubling, since the August 1913 paper explicitly states at one point, “It is to be remarked, too, that this is not a selected group of drops, but represents all the drops experimented upon during 60 consecutive days.” However, at another point in the paper he writes that the 58 drops reported are those “upon which a complete series of observations were made.” Furthermore, the margins of his notebook contain notes such as, “beauty publish” or “something wrong.”
Did Millikan deliberately disregard data that didn’t fit the results he wanted? Perhaps because he was under pressure from a rival and eager to make his mark as a scientist, Millikan misrepresented his data. Some have called this a clear case of scientific fraud. However, other scientists and historians have looked closely at his notebooks, and concluded that Millikan was striving for accuracy by reporting only his most reliable data, not trying to deliberately mislead others. For instance, he rejected drops that were too big, and thus fell too quickly to be measured accurately with his equipment, or too small, which meant they would have been overly influenced by Brownian motion. Some drops don’t have complete data sets, indicating they were aborted during the run.
It’s difficult to know today whether Millikan intended to misrepresent his results, though some scientists have examined Millikan’s data and calculated that even if he had included all the drops in his analysis, his measurement for the elementary charge would not have changed much at all.
Join your Society
If you embrace scientific discovery, truth and integrity, partnership, inclusion, and lifelong curiosity, this is your professional home.

COMMENTS
Millikan's experiment is based on observing charged oil droplets in free fall and the presence of an electric field. A fine mist of oil is sprayed across the top of a perspex cylinder with a small 'chimney' that leads down to the cell (if the cell valve is open). The act of spraying will charge some of the released oil droplets through friction ...
Milliken's Oil Drop Experiment Calculation. F up = F down. F up = Q . F down = m.g. Q is an electron's charge, E is the electric field, m is the droplet's mass, and g is gravity. One can see how an electron charge is measured by Millikan. Millikan found that all drops had charges that were 1.6x 10 -19 C multiples.
Millikan oil-drop experiment, first direct and compelling measurement of the electric charge of a single electron.It was performed originally in 1909 by the American physicist Robert A. Millikan, who devised a straightforward method of measuring the minute electric charge that is present on many of the droplets in an oil mist. The force on any electric charge in an electric field is equal to ...
Millikan's setup for the oil drop experiment. The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment took place in the Ryerson Physical Laboratory at the University of Chicago. Millikan received the Nobel Prize in Physics in 1923.. The experiment entailed observing tiny ...
The Millikan Oil-Drop Experiment HISTORY The year is 1911, and you are taking a physics course. Your professor is Robert Millikan. Professor Millikan has you and your classmates doing a lab experiment to measure e the magnitude of the charge of an electron, as well as to determine if charge is quantized (in other
The Millikan Oil Drop Experiment. Robert Millikan's oil drop experiment measured the charge of the electron. The experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. The choice of oil was important because most oils would evaporate under the heat of the light source, causing the drop to change mass ...
In Millikan's experiments ρ oil was 919.9 kg m -3 and ρ air was 1.2 kg m -3. The precision of the density of air is much less vital than that of the oil. The gravitational field g varies geographically, partly because of fluctuations in the earth's density and partly because of the eath's rotation. In Chicago g = 9.803 kg s -2.
This chemistry video for Grade 10-11 students demonstrates R. A. Millikan's oil drop experiment to calculate the charge of an electron.
This experiment first described in 1913, is based on the fact that different forces act on an electrically charged oil drop moving in the homogeneous electric field of a plate capacitor (Figure 1). Going through the capillary of the atomizer, the oil droplets acquire electric charge due to friction. The effect is known as triboelectric charging.
Robert Millikan and the Oil Drop Experiment Physics 401 4 ROBERT ANDREWS MILLIKAN 1868-1953 University of Chicago The Nobel Prize in Physics 1923. Robert A. Millikan "for his work on the elementary charge of electricity and on the photoelectric effect". Moved to Caltech in 1921.
Millikan Oil Drop Data Analysis: The experiment consists of raising a tiny, electrically charged oil drop in an electric field and then lowering it again. To raise it you apply a constant electric field on the drop that forces it upward. To lower the drop you can either turn off the electric field and just let it fall or you can reverse the ...
Measuring of the charge of the electron. Oil drop experiment. Robert A. Millikan.. (1909). e=1.5924(17)×10−19. Shot noise experiment. First proposed by Walter H. Schottky. In terms of the Avogadro constant and Faraday constant =. F- Faraday constant, NA- Avagadro constant. Best.
This video covers the famous Millikan experiment, determining the charge of an electron. Done in collaboration with Simon Crook (Crooked Science) and Tom Gor...
The Experiment. Devised by Robert A. Millikan and Harvey Fletcher, the Millikan Oil Drop Experiment is conducted in a chamber and is a method of measuring the electric charge of a single electron. To elaborate, this chamber contains an atomizer, a microscope, a light source, and two parallel metal plates. These metal plates obtain a negative ...
To see all my Chemistry videos, check outhttp://socratic.org/chemistryHow did scientists discover how much negative charge an electron had? Robert Millikan a...
These are separated by 12 mm and there is a potential difference of 1250 V across them. Calculate the charge of the oil drop. Answer: Step 1: List the known quantities: Mass, m = 5.1 × 10 -15 kg. Separation of plates, d = 12 mm. Potential difference, V = 1250 V. Acceleration due to gravity, g = 9.81 m s -2. Step 2: Recall the condition for a ...
Description. This simulation is a simplified version of an experiment done by Robert Milliken in the early 1900s. Hoping to learn more about charge, Milliken sprayed slightly ionized oil droplets into an electric field and made observations of the droplets. When the voltage is zero and the run button is pressed, the drop will fall due to the ...
Robert Millikan. Oil drop experiment The Nobel Prize in Physics 1923. Robert A. Millikan "for his work on the elementary charge of electricity and on the photoelectric effect". ROBERT ANDREWS MILLIKAN 1868-1953 22nd of March, 1868, Morrison, Ill University of Chicago 9/23/2013 4. Robert Millikan. Oil drop experiment
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron. Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general. Oil Drop Experiment.
The experiment initially allows the oil drops to fall between the plates in the absence of the electric field. They accelerate first due to gravity, but gradually the oil droplets slow down because of air resistance. The Millikan oil drop experiment formula can be given as below. Fup = Q ⋅ E Fdown = m. Where Q is an electron's charge, m is ...
Robert Millikan. Robert Millikan's famous oil drop experiment, reported in August 1913, elegantly measured the fundamental unit of electric charge. The experiment, a great improvement over previous attempts to measure the charge of an electron, has been called one of the most beautiful in physics history, but is also the source of allegations ...
For example, if a drop was too small, it was rejected for inaccuracy because it was affected by Stoke's law of viscosity in air. If a drop was too big, it would fall at a velocity too fast to record the time it took to drop accurately. In other words, Millikan was creating constant variables by disregarding these drops.
In this video, I discuss Milliken's oil-drop experiment, which aimed to determine the charge on a single electron. The experiment involved two parallel plate...