- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School

Blowing Up Balloons Respiration Style
June 18, 2012 By Emma Vanstone 5 Comments
We’ve talked about respiration before when we made bread and used yeast to make the dough rise. Blowing up a balloon with yeast is another very easy experiment to demonstrate respiration in action and is quicker than making bread if you are short of time.

What is respiration?
Respiration is a chemical reaction which occurs in animal and plant cells. It releases energy from glucose. Aerobic respiration needs oxygen, but anaerobic respiration doesn’t need oxygen.
Anaerobic respiration produces less energy than aerobic respiration. It occurs in humans when not enough oxygen reaches muscle cells ( for example, during hard exercise ). Bacteria and other microorganisms can also use anaerobic respiration, and yeast actually carry out an anaerobic process called fermentation .
Respiration occurs in the mitochondria of cells. You can find out more about mitochondria by making a model of a cell .
Blow up a balloon with yeast
A small clear drinks bottle
A packet of dried yeast
1 teaspoon of sugar

Instructions
1. Blow the balloon up a few times to give it some stretch. This just makes it easier for the experiment to work.
2. Fill the small bottle about 3cm full of warm water.
3. Add the yeast and 1 teaspoon of sugar.
4. Place the balloon over the open top so no air can escape.
5 Over the next half an hour, watch what happens. (Obviously, do other stuff and come back, it may be a little boring to actually watch it for half an hour!)
Yeast and Respiration
Yeast is a living organism. In order for it to survive, it needs to make energy. In its dried form, the yeast is dormant, but as soon as you provide it with warmth, water and sugar (its food), it ‘wakens’ and becomes active. The yeast uses the sugar (glucose) and oxygen from the bottle to make water, energy and carbon dioxide. Carbon dioxide is a gas, and this is what you see filling the balloon.
Remember, yeast can respire anaerobically when there’s not enough oxygen for aerobic respiration.
Fermentation
Glucose -> ethanol and carbon dioxide + energy
Aerobic Respiration Equation
Glucose + Oxygen –> Carbon Dioxide + Water + energy

The image is taken from Snackable Science which contains SEVENTY fun edible experiments and investigations!
Science concepts
Respiration
Contains affiliate links
Last Updated on May 3, 2023 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
June 18, 2012 at 3:04 pm
Oooh I like this one a lot! I am storing them all up for rainy days but I’ll get to this one quite quickly!
June 18, 2012 at 6:32 pm
What a cool project! Do the balloons float, then, like helium?
June 21, 2012 at 3:21 am
That’s so cool! We love everything science! My kids will love this!
June 25, 2012 at 8:14 pm
Brilliant experiment!!!! The kids will love it!
Thanks for sharing on Kids Get Crafty!
Maggy & Alissa
March 31, 2013 at 9:18 am
all the experiments simple and kids could easily understand the concepts behind it.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Want to adapt books like this? Learn more about how Pressbooks supports open practices.
8 Chapter 8 – Respiration
Respiration by yeast.
During respiration, yeast undergo metabolic processes to obtain energy from the breakdown of sugars. However, yeast can only metabolize certain types of sugars. In order for yeast to utilize a particular sugar as a food source, it needs to have specific transport mechanisms to bring the sugar molecules into its cells. Additionally, the yeast must possess the necessary enzymes capable of breaking down the chemical bonds in the sugar molecules in a way that can be used for energy production.
Among the various sugars, glucose is an essential source of energy for all living organisms, including yeast. Yeast can metabolize glucose through two different pathways: aerobic respiration and anaerobic fermentation. In aerobic respiration , yeast utilize oxygen to break down glucose molecules completely, resulting in the production of carbon dioxide (CO 2 ) and water (H 2 O) as byproducts. This process is highly efficient and yields a larger amount of energy in the form of ATP (adenosine triphosphate).
On the other hand, yeast can also carry out anaerobic respiration in the absence of oxygen. This process, known as fermentation, allows yeast to partially break down glucose molecules, resulting in the production of ethanol (alcohol) and carbon dioxide as byproducts. Although fermentation provides yeast with energy, it is less efficient compared to aerobic respiration.
In this lab, the objective is to investigate the ability of yeast to metabolize different sugars and observe respiration rate . The four sugars being tested are glucose, sucrose , fructose , and lactose . The experiment involves using a CO 2 Gas Sensor to measure the production of carbon dioxide by yeast as they respire using these sugars. The production of carbon dioxide indicates the metabolic activity of the yeast and provides insight into their ability to utilize the tested sugars as a food source.
By observing the rate and amount of carbon dioxide produced by the yeast when exposed to each sugar, it is possible to determine which sugars can be effectively metabolized by the yeast. This information helps in understanding the metabolic preferences and capabilities of yeast in utilizing different sugars for energy production.
- Respiration rate
Objectives
- Use a C0 2 Gas Sensor to measure concentrations of carbon dioxide.
- Determine the rate of respiration by yeast while using different sugars.
- Determine which sugars can be used as a food source by yeast.
- Vernier LabQuest 2 device
- Water bath @ 38-40 °C
- Vernier C02 Gas Sensor (2)
- Test tube rack
- Yeast Suspension*
- Deionized water
- 10x100mm test tubes (5)
- 5% Glucose, Sucrose, Lactose, and Fructose sugar solutions
- Disposable pipettes OR p-1000 Micropipettes with tips
- 250ml respiration chamber or Erlenmeyer flask (5)
*Stock solution of yeast: 7g of yeast (1 packet) in 100 mL water prepared fresh for the class, placed in 38-40 °C water bath for 10 minutes.
Pre-Assessment
1. What is the purpose of investigating the ability of yeast to metabolize different sugars in the lab? 2. How does yeast obtain energy from the breakdown of sugars during respiration? 3. What are the specific requirements for yeast to utilize a particular sugar as a food source? 4. Which sugar is considered an essential source of energy for all living organisms, including yeast? 5. What are the two pathways through which yeast can metabolize glucose? 6. Describe the byproducts produced during aerobic respiration of glucose by yeast. 7. What is the difference between aerobic respiration and anaerobic fermentation in yeast? 8. How does the efficiency of energy production differ between aerobic respiration and anaerobic fermentation in yeast? 9. What is the role of the CO 2 Gas Sensor in the lab experiment? 10. How can observing the rate and amount of carbon dioxide produced by yeast when exposed to different sugars help determine their metabolic preferences and capabilities?
- Prepare a water bath for the yeast. A water bath is simply a large reservoir of water at a certain temperature. This ensures that the yeast will remain at a constant and controlled temperature. Make sure the digital water bath has several inches of water in the basin. Turn the water bath on and set the temperature at 38-40°C. Monitor the temperature of the water bath during the experiment.
- Connect the C0 2 Gas Sensor to the LabQuest 2 device: Insert the plug into the CHI port at the left side of the device. Set the sensor switch to low (0 – 10,000 ppm) setting. Turn on the LabQuest 2 by pressing the button at the top left of the device.
- To clear any unwanted data from the device before beginning the experiment, tap ‘File’, then select ‘New’ from the drop down menu. When prompted, tap ‘Discard’.
- Obtain five test tubes and label them G, S, F, L, and W.
- Place 3 mL of the glucose solution in test tube G.
- Place 3 mL of the sucrose solution in test tube S.
- Place 3 mL of the fructose solution in test tube F.
- Place 3 mL of the lactose solution in test tube L.
- Place 3 mL of deionized water in test tube W.
- Obtain 15 mL of the yeast suspension. Gently swirl the yeast suspension to mix the yeast that settles to the bottom.
- Put 2 mL of the yeast suspension into the test tube labeled G (glucose). Gently swirl the test tube to mix the yeast into the solution.
- Set the test tube into the water bath and incubate for 10 minutes.
- When incubation is finished, use a pipet to place 3 mL of the solution from test tube G into the 250 mL respiration chamber or flask. Note the temperature of the water bath and record as the actual temperature in Table 1.
- Quickly place the shaft of the C0 2 Gas Sensor in the opening of the respiration chamber or flask. Gently twist the stopper on the shaft of the C0 2 Gas Sensor into the chamber opening. Do not twist the shaft of the C0 2 Gas Sensor or it could be damaged.
- Begin measuring the carbon dioxide concentration by tapping the green arrow ( at the bottom left of the screen. Collect data for 4 minutes (240 seconds), then press the red square to stop data collection. Pressing the arrow button on the right of the device will also start and stop data collection.
- A graph of C0 2 production over time will be displayed. Move your data to a stored run. To do this, tap on the filing cabinet icon at the top right of the screen.
- When data collection has finished, remove the C0 2 Gas Sensor from the respiration chamber. Use a notebook or notepad to fan air across the openings in the probe shaft of the C0 2 Gas Sensor for 1 minute.
- Repeat Steps 7 – 13 for the other four test tubes. Each run will be graphed a different color with a different shape for the data points (square, triangle, circle). It is a good idea to write down which graph represents which sugar.
- When data for all five tubes has been collected and stored, tap the screen next to the filing cabinet icon where it says ‘Run’. Select ‘All Runs’. The graphs for each sugar tested will be displayed.
- Tap ‘Analyze’ from the top of the screen.
- Select ‘Curve Fit’ from the drop-down menu. Select the square for the first sugar tested.
- Tap the arrow next to ‘Choose Fit’ and select ‘Linear’. The formula for a best fit line will appear (y = mx + b).
- RECORD THE SLOPE OF THE LINE (m) AS THE RATE OF RESPIRATION IN TABLE 1. The rate of respiration given by m on the graph is in ppm/s. This should be converted to ppm/min for Table 1.
- Tap OK at the bottom right. This displays the best fit line on the graph.
- Determine the slope of the line (m) for each of the sugars by repeating steps 1 through 5, selecting the next square for the next sugar tested.
- Name (Bench #) and save your file
- Insert your USB (if LabQuest2 does not register the USB, ensure the USB is inserted properly)
- Highlight your named file and continue.
- It is now safe to remove the USB once exporting is complete.
- Upload your file to a device and make sure the entire group has a copy. You can use this file for data manipulation.
- Upload the data into the Respiration discussion board on Canvas.
- Clear the data by tapping ‘File’ and selecting ‘New’. Tap ‘Discard’ when prompted.
- Press the button on the device with the house icon.
- Select (System’, then ‘Shut Down’.
- Tap ‘OK’ when prompted.
- Return sensors and LabQuest 2 to the cart. Wash all glassware.
| Sugar Tested | Temperature (°C) | Respiration Rate (ppm/min) |
| Glucose | ||
| Sucrose | ||
| Fructose | ||
| Lactose | ||
| Water (control) |
| Sugar Tested | Respiration Rate (ppm/min) |
| Glucose | |
| Sucrose | |
| Fructose | |
| Lactose | |
| Water |
DATA ANALYSIS & CRITICAL THINKING QUESTIONS
- (Optional) When all other groups have posted their results on the board, calculate the average rate of respiration for each solution tested. Record the average rate values in Table 2.
- Make a bar graph of rate of respiration vs. sugar type. The rate values should be plotted on the y-axis, and the sugar type on the x-axis. Use the rate values from Table 1.
- Considering the results of this experiment, do yeast equally utilize all sugars? Explain.
- Hypothesize why some sugars were not metabolized while other sugars were.
- Why do you need to incubate the yeast before you start collecting data?
- Yeast live in many different environments. Make a list of some locations where yeast might naturally grow. Estimate the possible food sources at each of these locations.
Licenses and Attributions
Yeast is a type of fungus belonging to the kingdom Fungi. It is a single-celled microorganism that plays a significant role in various biological processes, especially in the context of fermentation and baking. Yeast cells are eukaryotic, meaning they possess a true nucleus and other membrane-bound organelles.
Yeast is widely used in the food and beverage industry, particularly in baking and brewing. It has been utilized by humans for thousands of years for the fermentation of sugars, which results in the production of carbon dioxide, ethanol, and other metabolic byproducts. This fermentation process is essential in the leavening of bread, where carbon dioxide gas produced by yeast causes the dough to rise, resulting in a light and fluffy texture.
The most commonly used species of yeast in baking and brewing is Saccharomyces cerevisiae. It has the ability to metabolize various sugars, including glucose, sucrose, fructose, and lactose, through the process of respiration. Yeast cells break down these sugars to obtain energy in the form of ATP (adenosine triphosphate), which is the primary energy currency of cells.
Apart from its role in fermentation, yeast also serves as a model organism in biological research. Due to its simple and well-studied genetics, yeast has provided valuable insights into various cellular processes and molecular mechanisms. Researchers have used yeast to study fundamental biological phenomena, such as cell cycle regulation, DNA replication, protein synthesis, and aging.
Yeast cells reproduce asexually through a process called budding, where a small bud or protrusion forms on the parent cell and eventually separates to become a new individual. Under certain conditions, yeast can also undergo sexual reproduction, involving the fusion of two yeast cells and the exchange of genetic material.
In summary, yeast is a single-celled fungus that plays a vital role in fermentation, baking, brewing, and scientific research. Its ability to metabolize sugars and produce carbon dioxide and ethanol has made it an indispensable microorganism in the food and beverage industry. Additionally, yeast's genetic simplicity and biological characteristics have made it a valuable model organism for understanding fundamental biological processes.
Glucose is a simple sugar and one of the most important carbohydrates in biological systems. It is a primary source of energy for living organisms and plays a crucial role in cellular metabolism. Glucose is a monosaccharide, which means it consists of a single sugar molecule. Its chemical formula is C6H12O6.
Glucose is found in various forms and sources in nature. Some common examples include:
1. Blood sugar: Glucose is the main sugar found in the bloodstream of animals, including humans. It is transported through the bloodstream to provide energy to cells throughout the body.
2. Plant sap: Glucose is present in the sap of plants, which serves as a nutrient-rich fluid that transports sugars and other substances within the plant's vascular system.
3. Fruits: Many fruits contain glucose, often in combination with other sugars like fructose. Fruits such as grapes, bananas, apples, and oranges are examples of glucose-containing fruits.
4. Honey: Bees produce honey by converting nectar, a sugary liquid found in flowers, into a concentrated solution. Honey contains glucose as well as other sugars.
5. Starches: Glucose molecules are the building blocks of starch, a polysaccharide found in various plant-based foods. Starchy foods like potatoes, rice, wheat, and corn contain glucose in the form of long chains of starch molecules.
6. Sweeteners: Glucose is used as a sweetener in various food products. It is commonly found in syrups like corn syrup and high-fructose corn syrup, which are widely used in the food industry.
7. Metabolism: Glucose is produced through the breakdown of more complex carbohydrates, such as glycogen (the storage form of glucose in animals) and starches, during digestion. It is then used by cells as a fuel source through processes like glycolysis and cellular respiration.
Glucose is an essential energy source for organisms and serves as a fundamental component in many biological processes. Its availability and regulation in the body are crucial for maintaining normal physiological functions.
Aerobic metabolism of sugar refers to the process by which cells, including yeast cells, break down sugar molecules in the presence of oxygen to produce energy. This process is also known as cellular respiration and occurs in several sequential steps.
The first step in aerobic metabolism is glycolysis, which takes place in the cytoplasm of the cell. During glycolysis, a molecule of glucose, a six-carbon sugar, is converted into two molecules of pyruvate, a three-carbon compound. This process involves the breakdown of glucose into smaller molecules, releasing a small amount of ATP (adenosine triphosphate) and NADH (nicotinamide adenine dinucleotide, a coenzyme) as byproducts.
After glycolysis, if oxygen is available, the pyruvate molecules produced are transported into the mitochondria, the powerhouses of the cell. In the mitochondria, the pyruvate molecules undergo further oxidation in a process called the citric acid cycle, also known as the Krebs cycle or TCA (tricarboxylic acid) cycle. The citric acid cycle involves a series of chemical reactions that break down the pyruvate molecules, releasing more ATP, NADH, and FADH2 (flavin adenine dinucleotide, another coenzyme).
The NADH and FADH2 molecules produced during glycolysis and the citric acid cycle play a crucial role in the final step of aerobic metabolism: oxidative phosphorylation. Oxidative phosphorylation occurs in the inner mitochondrial membrane and involves the transfer of electrons from NADH and FADH2 to a series of protein complexes known as the electron transport chain (ETC). As electrons pass through the ETC, they release energy that is used to pump protons (H+) across the inner mitochondrial membrane, creating an electrochemical gradient.
The electrochemical gradient created by the ETC drives the synthesis of ATP through a process called chemiosmosis. ATP synthase, an enzyme located in the inner mitochondrial membrane, utilizes the energy from the proton gradient to produce ATP. This process is known as oxidative phosphorylation because it couples the phosphorylation of ADP (adenosine diphosphate) to form ATP with the oxidation of NADH and FADH2.
Overall, aerobic metabolism of sugar provides the most efficient way to produce energy in cells. It yields a large amount of ATP compared to anaerobic processes like fermentation. By utilizing oxygen, cells can fully break down sugar molecules, extracting maximum energy from them and generating carbon dioxide and water as byproducts. This process is essential for the functioning and survival of cells, including yeast cells, in oxygen-rich environments.
Anaerobic metabolism of sugar, also known as fermentation, is a metabolic pathway that allows cells to generate energy from sugar molecules in the absence of oxygen. Unlike aerobic metabolism, which occurs in the presence of oxygen and yields more energy, anaerobic metabolism is less efficient and produces fewer ATP molecules.
There are several types of anaerobic fermentation, but one of the most common forms is alcoholic fermentation, which occurs in yeast and some bacteria. In this process, glucose or other sugars are converted into ethanol (alcohol) and carbon dioxide.
The first step of anaerobic metabolism is glycolysis, which takes place in the cytoplasm of the cell. During glycolysis, a molecule of glucose is broken down into two molecules of pyruvate, similar to the process in aerobic metabolism. This step releases a small amount of ATP and NADH.
In the absence of oxygen, pyruvate molecules undergo further conversion through fermentation. In the case of alcoholic fermentation, pyruvate is decarboxylated, meaning it loses a carbon dioxide molecule, to form acetaldehyde. This reaction is catalyzed by the enzyme pyruvate decarboxylase. Simultaneously, NADH is oxidized back to NAD+ by transferring its electrons to the acetaldehyde, producing NAD+ and NADH. Acetaldehyde then undergoes a second reaction, catalyzed by the enzyme alcohol dehydrogenase, where it is reduced by NADH to produce ethanol. This final step regenerates NAD+ for further glycolysis to continue.
The overall process of anaerobic metabolism of sugar is less efficient than aerobic metabolism because it produces only a small amount of ATP. In addition to ethanol, carbon dioxide is also produced as a byproduct. This is why during alcoholic fermentation, we observe the release of carbon dioxide bubbles.
Anaerobic metabolism is important for organisms that live in environments with low oxygen availability. It allows them to continue producing energy even when oxygen is limited or absent. For example, yeast cells undergo anaerobic metabolism when fermenting sugars in the absence of oxygen, which is utilized in the production of alcoholic beverages and bread-making.
It's important to note that different organisms can undergo other types of anaerobic fermentation, such as lactic acid fermentation in some bacteria and human muscle cells. These processes involve different intermediate compounds and produce lactic acid instead of ethanol as the end product.
Respiration rate refers to the rate at which an organism or a cell undergoes cellular respiration, which is the process of breaking down organic molecules, such as sugars, to produce energy in the form of ATP (adenosine triphosphate). In the case of yeast, respiration rate specifically refers to the rate at which yeast cells carry out the process of cellular respiration.
Yeast cells are capable of metabolizing sugars through aerobic respiration, which occurs in the presence of oxygen, or anaerobic respiration, which occurs in the absence of oxygen. Both forms of respiration involve the breakdown of sugar molecules to release energy.
During aerobic respiration, yeast cells convert glucose, sucrose, fructose, lactose, or other sugars into carbon dioxide (CO2) and water (H2O). This process involves a series of enzymatic reactions that occur in the mitochondria of yeast cells. Oxygen is used as the final electron acceptor in the electron transport chain, allowing for the efficient production of ATP.
In contrast, during anaerobic respiration, yeast cells can metabolize sugars without the presence of oxygen. Instead of utilizing oxygen as the final electron acceptor, yeast cells undergo a process called fermentation. In this process, sugar molecules are partially broken down, producing alcohol (typically ethanol) and carbon dioxide as byproducts. This type of respiration is commonly observed in yeast when they are in an oxygen-deprived environment, such as when fermenting fruits to produce alcoholic beverages or when used in baking to make bread rise.
The respiration rate of yeast can be measured by monitoring the production of carbon dioxide gas. As yeast cells metabolize sugars, they release carbon dioxide as a byproduct, which can be detected and quantified using various methods, such as gas sensors or gas chromatography. By measuring the rate of carbon dioxide production, researchers can assess the metabolic activity and respiration rate of yeast under different conditions, such as varying sugar concentrations, temperatures, or pH levels.
Overall, the respiration rate of yeast provides insights into its metabolic activity, energy production, and ability to utilize different sugars as energy sources. It is an important parameter to consider when studying yeast physiology, fermentation processes, or when using yeast in various applications, such as brewing, winemaking, or biofuel production.
Sucrose is a disaccharide composed of glucose and fructose molecules linked together. It is commonly known as table sugar and is one of the most widely used sweeteners in the world. The chemical formula of sucrose is C12H22O11.
Sucrose is found in various natural sources and is commonly used as a sweetening agent in food and beverages. Here are some common examples of where sucrose is found:
1. Sugarcane: Sucrose is abundantly found in sugarcane plants. Sugarcane juice is extracted from the stalks of the plant and processed to obtain crystalline sucrose, which is further refined to produce table sugar.
2. Sugar beet: Sugar beet is another major commercial source of sucrose. The sucrose content is extracted from the root of the sugar beet plant and processed similarly to sugarcane to obtain sugar.
3. Fruits: Sucrose is naturally present in many fruits, although in varying amounts. Fruits like mangoes, pineapples, oranges, and strawberries contain sucrose along with other sugars like glucose and fructose.
4. Processed foods: Sucrose is widely used as a sweetener in processed foods such as baked goods, candies, desserts, soft drinks, and sauces. It provides sweetness and enhances the flavor of these products.
5. Condiments: Some condiments and spreads, such as jams, jellies, and syrups, contain sucrose to add sweetness and improve the taste.
6. Confectionery: Chocolates, candies, and confectionery products often contain sucrose as a primary sweetener. It contributes to the desirable taste and texture of these treats.
7. Beverages: Many beverages, including soda, energy drinks, and sweetened teas, contain sucrose for sweetening purposes.
Sucrose is widely consumed in the human diet and is a common ingredient in various culinary preparations. However, it is important to consume sucrose in moderation as excessive intake can contribute to health issues like obesity, dental problems, and metabolic disorders.
Fructose is a monosaccharide, or simple sugar, that is naturally occurring in many fruits, vegetables, and sweeteners. It is the sweetest of all naturally occurring sugars and has the same chemical formula as glucose (C6H12O6), but with a different arrangement of atoms.
Here are some key points about fructose and common examples of where it is found:
1. Natural Sources: Fructose is found in varying amounts in a wide range of fruits and vegetables. Some examples include:
- Fruits: Fructose is abundant in fruits such as apples, pears, grapes, cherries, peaches, and mangoes. It contributes to the natural sweetness of these fruits.
- Honey: Honey contains a mixture of fructose and glucose, with fructose being the predominant sugar. Bees convert nectar from flowers into honey, which is a concentrated source of fructose.
- Agave: Agave syrup, derived from the agave plant, is a popular natural sweetener that primarily consists of fructose. It is commonly used as an alternative to table sugar.
2. Processed Foods and Beverages: Fructose is also used as an added sweetener in various processed foods and beverages. High-fructose corn syrup (HFCS) is a common sweetener used in soft drinks, processed snacks, cereals, and baked goods. It is produced by enzymatically converting glucose from cornstarch into fructose, resulting in a sweeter and more economical alternative to sucrose (table sugar).
3. Sweeteners: Crystalline fructose, which is a highly pure form of fructose, is used as a sweetening agent in some food products. It is sweeter than sucrose and can be found in certain beverages, desserts, and low-calorie or sugar-free products.
It is worth noting that excessive consumption of fructose, particularly in the form of added sugars like high-fructose corn syrup, has been associated with potential health risks. High intake of fructose from processed foods and beverages has been linked to increased risk of obesity, type 2 diabetes, and metabolic disorders. As with any sugar, moderation is key when consuming fructose as part of a balanced diet.
Lactose is a disaccharide composed of two sugar molecules, glucose and galactose, linked together. It is commonly referred to as milk sugar because it is the primary sugar found in milk and dairy products. Lactose is unique in that it is primarily found in mammalian milk, including human breast milk.
Here are some key points about lactose and common examples of where it is found:
1. Milk and Dairy Products: Lactose is abundantly present in milk from various mammalian species, including cows, goats, and sheep. It serves as the main carbohydrate source in milk. Dairy products such as yogurt, cheese, butter, and ice cream also contain lactose, although the concentration may vary depending on the specific product.
2. Human Breast Milk: Lactose is an essential component of human breast milk, providing a source of energy for infants. It plays a crucial role in the development and growth of newborns. Human breast milk typically contains higher levels of lactose compared to the milk of other mammalian species.
3. Lactose-Containing Food Products: Lactose is sometimes used as an ingredient in processed food products, especially those aimed at individuals who are lactose intolerant. These products may include lactose-reduced or lactose-free dairy alternatives, lactose-free milk, lactose-free ice cream, and lactose-free yogurts.
4. Medications and Supplements: Lactose is also used as an excipient in some medications and dietary supplements. It can act as a filler or a binding agent in tablet or capsule formulations. Individuals with lactose intolerance or milk allergies should be cautious about consuming such products and may need to seek alternatives.
Lactose intolerance is a common condition where the body lacks sufficient amounts of the enzyme lactase, which is responsible for breaking down lactose into glucose and galactose for absorption in the small intestine. This leads to digestive symptoms, such as bloating, gas, and diarrhea, after consuming lactose-containing foods or beverages. As a result, individuals with lactose intolerance often need to limit or avoid foods high in lactose or use lactase supplements to aid in digestion.
It's important to note that lactose is not naturally present in non-dairy plant-based beverages like soy milk, almond milk, or coconut milk. These products are typically made by grinding or blending the respective plant material with water, without the addition of lactose.
Biology I Cellular Processes Laboratory Manual by The authors & Hillsborough Community College is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
- The Open University
- Accessibility hub
- Guest user / Sign out
- Study with The Open University
My OpenLearn Profile
Personalise your OpenLearn profile, save your favourite content and get recognition for your learning
About this free course
Become an ou student, download this course, share this free course.

Start this free course now. Just create an account and sign in. Enrol and complete the course for a free statement of participation or digital badge if available.
3.1.3 Yeast experiment explained

You’ve seen the results of the yeast experiment, but what do these results mean?
Yeasts are microscopic, single-celled organisms, and are a type of fungus that is found all around us, in water, soil, on plants, on animals and in the air. Like all organisms, when yeasts are put in the right type of environment they will thrive; growing and reproducing.
Your experiments were designed to help you identify which environment promotes the most yeast growth. The first three glasses in your experiment contained different temperature environments (cold water, hot water and body temperature water). At very low temperatures the yeast simply does not grow but it is still alive – if the environment were to warm up a bit, it would gradually begin to grow. At very high temperatures the cells within the yeast become damaged beyond repair and even if the temperature of that environment cooled, the yeast would still be unable to grow. At optimum temperatures the yeast thrives.
Your third and fourth glasses both contained environments at optimum temperature (body temperature) for yeast growth, the difference being, the fourth glass was sealed. The variable between these two experiments was the amount of available oxygen. You may have been surprised by your results here, thinking that a living organism in an environment without oxygen cannot survive? However, you should have found that yeast grew pretty well in both experiments.
To understand why yeast was able to thrive in both conditions we need to understand the chemical process occurring in each glass during the experiment. In the three open glasses, oxygen is readily available, and from the moment you added the yeast to the sugar solution it began to chemically convert the sugar in the water and the oxygen in the air into energy, water, and carbon dioxide in a process called aerobic respiration.
Yeast is a slightly unusual organism – it is a ‘facultative anaerobe’. This means that in oxygen-free environments they can still survive. The yeast simply switches from aerobic respiration (requiring oxygen) to anaerobic respiration (not requiring oxygen) and converts its food without oxygen in a process known as fermentation. Due to the absence of oxygen, the waste products of this chemical reaction are different and this fermentation process results in carbon dioxide and ethanol.
Depending on how long you monitored your experiment for and how much space your yeast had to grow you may have noticed that, with time, the experiment sealed with cling film slowed down. This is for two reasons; firstly because less energy is produced by anaerobic respiration than by aerobic respiration and, secondly, because the ethanol produced is actually toxic to the yeast. As the ethanol concentration in the environment increases, the yeast cells begin to get damaged, slowing their growth.
The ethanol produced is a type of alcohol, so it is this process that allows us to use it to make beer and wine. When used in bread making, the yeast begins by respiring aerobically, the carbon dioxide from which makes the bread rise. Eventually the available oxygen is used up, and the yeast switches to anaerobic respiration producing alcohol and carbon dioxide instead. Do not worry though; this alcohol evaporates during the baking process, so you won’t get drunk at lunchtime from eating your sandwiches.

- Physiology Videos
- Anatomy Videos
- High School Biology Videos
Cellular Respiration in Yeast Lab
This lab explores the concepts of Cellular Respiration and Fermentation in yeast . Yeast do Alcoholic Fermentation and one of the byproducts is Carbon Dioxide. When you bake bread with yeast, Carbon dioxide is produced, which forms bubbles in the dough, causing the dough to rise. The heat kills the yeast and the bubble pockets lighten the bread.
In this lab, you can actually see fermentation happening by measuring “bubble production”. The tricky part of this lab is setting it up. So I put together a little video to show you exactly how to do that. Special thanks to my Student Worker Brian Powers for helping me film the video. He did a pretty good job – Smooth zooming and all. Enjoy!
Video Introduction
[flashvideo file=http://leslie-samuel.s3.amazonaws.com/YeastRespiration.flv image=https://interactivebiology.com/images/cellularresp.jpg /]

Lab Handout
And here’s the actual lab. You can download it by clicking on the download link on the top left of the document. You will need Adobe Acrobat or another pdf viewer in order to download it and see it on your computer.
Sample Results
The following picture was taken approximately 45 minutes after the test tubes were placed in an incubator at 30 degrees Celcius. You can see that there’s CO2 production in both samples with sugar and none in the control (click image to enlarge).
You may also like
Every human would die without this cell, how to do an ecg at home | wellue 24 hour ecg recorder, t-cell development and maturation.
Page [tcb_pagination_current_page] of [tcb_pagination_total_pages]
Leave a Reply
You must be logged in to post a comment.
i like your way of presentation
i like it but u should present it with examples
hi i am nursing st of cardiology wants to study in easy way
ya ilike it
I liked your method of teaching
Get in touch
Get a FREE Copy of The Cardiac Cycle Made EASY!

Stop struggling to understand the Cardiac Cycle TODAY!
Get a free copy of The Cardiac Cycle Made EASY!!


Reading & Math for K-5
- Kindergarten
- Learning numbers
- Comparing numbers
- Place Value
- Roman numerals
- Subtraction
- Multiplication
- Order of operations
- Drills & practice
- Measurement
- Factoring & prime factors
- Proportions
- Shape & geometry
- Data & graphing
- Word problems
- Children's stories
- Leveled Stories
- Sentences & passages
- Context clues
- Cause & effect
- Compare & contrast
- Fact vs. fiction
- Fact vs. opinion
- Main idea & details
- Story elements
- Conclusions & inferences
- Sounds & phonics
- Words & vocabulary
- Reading comprehension
- Early writing
- Numbers & counting
- Simple math
- Social skills
- Other activities
- Dolch sight words
- Fry sight words
- Multiple meaning words
- Prefixes & suffixes
- Vocabulary cards
- Other parts of speech
- Punctuation
- Capitalization
- Narrative writing
- Opinion writing
- Informative writing
- Cursive alphabet
- Cursive letters
- Cursive letter joins
- Cursive words
- Cursive sentences
- Cursive passages
- Grammar & Writing
Breadcrumbs
Yeast Respiration Science Experiment
This simple experiment allows you to investigate yeast respiration.
These are the materials you need:
- Two small plastic bottles or jars
- Rubber band
- Thermometer

Methodology
Now, proceed as follows:
Add one teaspoon of sugar and one teaspoon of dry yeast to a small plastic bottle or jar.
Add about 100 ml of warm water to the bottle and stir until the yeast and sugar are dissolved.
Attach a balloon to the top of the bottle using a rubber band.
Repeat steps 1-3 with a second bottle, but do not add any sugar to this bottle.
Place both bottles in a warm place (around 30-35°C) and wait for 10-15 minutes.
Observe the balloons on both bottles. Which one has inflated more?
Use a thermometer to measure the temperature inside both bottles. Were they both at the same temperature?

Explanation
Yeast can respire in the presence or absence of oxygen. When yeast is added to warm water and sugar, it will begin to respire and release carbon dioxide gas as a waste product. The carbon dioxide gas will inflate the balloon attached to the bottle. The more the yeast respires, the more carbon dioxide is produced and the more the balloon inflates.
In the second bottle, where no sugar was added, the yeast does not have any glucose to respire. Therefore, no carbon dioxide gas is produced and the balloon remains uninflated.
By measuring the temperature inside both bottles, you can find out that the yeast is not affected by the temperature differences, and that the difference in balloon inflation is due to the presence or absence of glucose.
This content is available to members only.
Join K5 to save time, skip ads and access more content. Learn More
Yeast-Air Balloons
The purpose of any leavener is to produce the gas that makes bread rise. Yeast does this by feeding on the sugars in flour, and expelling carbon dioxide in the process.
While there are about 160 known species of yeast, Saccharomyces cerevisiae, commonly known as baker's yeast, is the one most often used in the kitchen. Yeast is tiny: Just one gram holds about 25 billion cells. That amount of fungi can churn out a significant amount of carbon dioxide, provided it has the simple sugars it uses as food. Fortunately, yeast can use its own enzymes to break down more complex sugars—like the granulated sugar in the activity below—into a form that it can consume.
Make a yeast-air balloon to get a better idea of what yeast can do.

Did You Know?
What do i need.
1 packet of active dry yeast
1 cup very warm water (105° F-115° F)
2 tablespoons sugar
a large rubber balloon
a small (1-pint to 1-liter) empty water bottle
Kids, please don t try this at home without the help of an adult.
What do I do

Stretch out the balloon by blowing it up repeatedly, and then lay it aside.
Add the packet of yeast and the sugar to the cup of warm water and stir.
Once the yeast and sugar have dissolved, pour the mixture into the bottle. You ll notice the water bubbling as the yeast produces carbon dioxide.
Attach the balloon to the mouth of the bottle, and set both aside.
Step 5: After several minutes, you ll notice the balloon standing upright. If you don t see anything happen, keep waiting. Eventually, the balloon will inflate.
What's going on.
As the yeast feeds on the sugar, it produces carbon dioxide. With no place to go but up, this gas slowly fills the balloon.
A very similar process happens as bread rises. Carbon dioxide from yeast fills thousands of balloonlike bubbles in the dough. Once the bread has baked, this is what gives the loaf its airy texture.
What Else Can I Try?
Try the same experiment, but this time use about a tablespoon of baking powder instead of yeast, and leave out the sugar. What differences do you notice? Which leavener takes longer to fill up the balloon?
Also, try the same experiment using hotter and colder water. Use a thermometer to measure the temperature of the water. At what temperature is the yeast most active? At what temperatures is it unable to blow up the balloon?

Carbon Dioxide Production

Procedures:
- Fill the six eudiometers with colored tap water (colored water is easier to read). Invert each in a 600mL or larger beaker on a ring stand.
- Create 10% solutions of each of the six sugars. (add 10.0g sugar to 90.0mL purified water) each in a 250mL Erlenmeyer flask. Label each flask.
- Assemble #5 stoppers with glass and rubber tubing.
- Measure six 1.0g portions of yeast. Place each portion in a different flask.
- Install the stopper apparatus into each test tube. Insert the unattached end of each rubber tube into the corresponding inverted eudiometer.
- Start the clock. Record the volume of carbon dioxide produced at 5 or 10 minute increments until no more gas can be measured.
- Graph data as indicated in the example below.
- Electronic Balance
- Weighing Dishes or Paper
- 6 x 600mL beakers or larger
- ring stands with clamps
- 100mL Graduated Cylinder
- 6 x 250mL Erlenmeyer Flasks
- 6 x single-hole #5 Rubber Stoppers
- 6 x 4" Glass Tubing
- 6 x 10" Lengths Rubber Tubing
- 6 x 100mL Eudiometers
- 540mL Purified Water
- Tap Water (for displacement)
- Food Coloring (optional)
Consumables:
- 2 x 7g Packages Dry Yeast
- 10g Dextrose (Glucose)
- 10g Levulose (Fructose)
- 10g Galactose
- 10g Sucrose
- 10g Maltose
- 10g Lactose
| Saccharide | Structure | |
|---|---|---|
It is interesting to note that, not only do these sugars look different, but they also have distinct odors.

Remember Me

Shop 2.8 Cell Respiration 2.8 Cell Respiration
Experiments.
Here are experiments our science specialists have selected to support the IB* topic.

Recommended
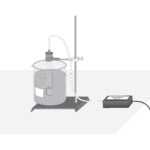
Respiration of Sugars by Yeast
Experiment #12A from Biology with Vernier
In this experiment, you will
- Use a CO 2 Gas Sensor to measure concentrations of carbon dioxide.
- Determine the rate of respiration by yeast while using different sugars.
- Determine which sugars can be used as a food source by yeast.
Sugar Fermentation
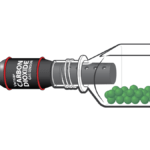
Experiment #12B from Biology with Vernier
- Use a Gas Pressure Sensor to measure the pressure change caused by carbon dioxide released during fermentation.
- Determine the rate of fermentation.
- Determine which sugars yeast can metabolize.
Recommended Inquiry
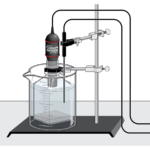
Cellular Respiration
Experiment #9 from Investigating Biology through Inquiry
In this Preliminary Activity, you will use a CO 2 Gas Sensor to determine the respiration rate of peas. After completing the Preliminary Activity, you will first use reference sources to find out more about peas, seeds, germination, and cell respiration before you choose and investigate a researchable question dealing with the cell respiration. Some topics to consider in your reference search are:
- germination
- cell respiration
- carbohydrates
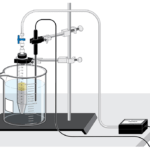
Sugar Metabolism with Yeast (Carbon Dioxide Gas)
Experiment #10A from Investigating Biology through Inquiry
In the Preliminary Activity, you will use a CO 2 Gas Sensor to determine the rate of respiration of glucose by yeast.
After completing the Preliminary Activity, you will first use reference sources to find out more about sugars, respiration, and yeast before you choose and investigate a researchable question dealing with the respiration of sugars by yeast. Some topics to consider in your reference search are:
- monosaccharides
- disaccharides
- polysaccharides
- aerobic respiration
- anaerobic respiration
Fermentation with Yeast
Experiment #11 from Investigating Biology through Inquiry
In this Preliminary Activity, you will use a Gas Pressure Sensor to monitor the pressure inside a conical tube as yeast metabolizes glucose anaerobically. When data collection is complete, you will perform a linear fit on the resultant graph to determine the fermentation rate.
After completing the Preliminary Activity, you will first use reference sources to find out more about sugar fermentation by yeast before you choose and investigate a researchable question dealing with fermentation. Some topics to consider in your reference search are:
- fermentation
- enzyme inhibitor
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
Pardon Our Interruption
As you were browsing something about your browser made us think you were a bot. There are a few reasons this might happen:
- You've disabled JavaScript in your web browser.
- You're a power user moving through this website with super-human speed.
- You've disabled cookies in your web browser.
- A third-party browser plugin, such as Ghostery or NoScript, is preventing JavaScript from running. Additional information is available in this support article .
To regain access, please make sure that cookies and JavaScript are enabled before reloading the page.

IMAGES
VIDEO
COMMENTS
This is another very easy experiment that demonstrates respiration in action and is quicker than the bread, if you are short of time. Yeast is a living organism. In order for it to survive it needs to make energy. In its dried form the yeast is dormant, but as soon as you provide it with warmth, water and Sugar (it's food) it 'wakens' and becomes active. The sugar (glucose) reacts with the ...
8 Chapter 8 - Respiration Respiration by Yeast BACKGROUND. During respiration, yeast undergo metabolic processes to obtain energy from the breakdown of sugars. However, yeast can only metabolize certain types of sugars. In order for yeast to utilize a particular sugar as a food source, it needs to have specific transport mechanisms to bring the sugar molecules into its cells.
This experiment uses a living organism to investigate the conditions under which life grows the best.(Part 8 of 10)Playlist link - http://www.youtube.com/pla...
The yeast simply switches from aerobic respiration (requiring oxygen) to anaerobic respiration (not requiring oxygen) and converts its food without oxygen in a process known as fermentation. Due to the absence of oxygen, the waste products of this chemical reaction are different and this fermentation process results in carbon dioxide and ethanol.
Objective: In this lab, students will use the respiration powers of yeast to blow balloons. This activity will reinforce the basic principles of respiration as a fundamental metabolic process for living organisms using yeast as a model. It will also explore how humans use this biological knowledge in everyday life. Material: balloons
Objective To observe the process of cellular respiration in live yeast. We will also observe how changing food concentrations can affect the rate of cellular respiration. Background Cellular respiration is the process of converting chemical energy (food) into ATP that the organism can use for its cellular processes.
This lab explores the concepts of Cellular Respiration and Fermentation in yeast. Yeast do Alcoholic Fermentation and one of the byproducts is Carbon Dioxide. When you bake bread with yeast, Carbon dioxide is produced, which forms bubbles in the dough, causing the dough to rise. The heat kills the yeast and the bubble pockets lighten the bread.
Yeast makes tiny gas bubbles in our in bread before it gets baked. This experiment demonstrates cellular respiration when the yeast consumes the sugar and releases carbon dioxide. Materials: A packet of yeast (available in the grocery store) A small, clean, clear, plastic bottle (16 oz. or smaller) 1 teaspoon of sugar
Add one teaspoon of sugar and one teaspoon of dry yeast to a small plastic bottle or jar. Add about 100 ml of warm water to the bottle and stir until the yeast and sugar are dissolved. Attach a balloon to the top of the bottle using a rubber band. Repeat steps 1-3 with a second bottle, but do not add any sugar to this bottle.
Yeast-Air Balloons. The purpose of any leavener is to produce the gas that makes bread rise. Yeast does this by feeding on the sugars in flour, and expelling carbon dioxide in the process. While there are about 160 known species of yeast, Saccharomyces cerevisiae, commonly known as baker's yeast, is the one most often used in the kitchen.
Remember, yeast is made of two glucose molecules. Glucose (aka dextrose) is a close second. Fructose is in third place. Interestingly, sucrose, made of glucose and fructose, does not perform well. Perhaps yeast do not have an enzyme to access sucrose's energy. Fructose, galactose, and lactose produced very little, if any cellular respiration in ...
In this lab, you will try to determine whether yeast are capable of metabolizing a variety of sugars. When yeast respire aerobically, oxygen gas is consumed and carbon dioxide, CO2, is produced. You will use a CO 2 Gas Sensor to monitor the production of carbon dioxide as yeast respire using different sugars. The four sugars that will be tested ...
respiration in yeast. In Part 1, a guided inquiry, students conduct and report on an experiment to determine the effect of temperature on respiration. In Part 2, an open-ended inquiry, students design, conduct, and report on a controlled experiment to determine how changing the type of food affects yeast respiration. Core Concepts
This lab, however, will not be focusing on aerobic respiration which requires oxygen. The lab investigates the rate of respiration in Saccharomyces Cerevisiae , commonly known as Baker's yeast. When it has run out of oxygen, yeast uses anaerobic respiration to break down glucose molecules and convert them to ATP. After, it starts producing ...
Method: Add a spatula of yeast to each of four conical flasks. Add one spatula of sugar to the first flask, two spatulas of sugar to the next flask, three spatulas to the third flask and finally, four spatulas of sugar to the fourth conical flask. Add 25ml of warm water to each of the flasks. Stir each flask thoroughly then proceed to cover ...
Use an optical dissolved oxygen sensor and a fast response temperature probe to investigate the effect of temperature on the cellular respiration rate of yea...
Step 1: Glycolysis. During the first step of cellular respiration, glucose, a simple sugar, enters the cell. The yeast uses the glucose and creates two ATP, two NADH (a molecule that carries ...
Respiration of Sugars by Yeast. Experiment #12A from Biology with Vernier. In this experiment, you will. Use a CO 2 Gas Sensor to measure concentrations of carbon dioxide. Determine the rate of respiration by yeast while using different sugars. Determine which sugars can be used as a food source by yeast. Recommended. Sugar Fermentation
For this experiment, you'll want to make sure your yeast has the reactants needed for the experiment to occur. As you go through the experiment keep in mind what the yeast is producing which will fill up the balloon. First, we are going to establish a baseline of how yeast cellular respiration effects the balloon.
SynopsisimageChemical cell-to-cell communication in the fission yeast Schizosaccharomyces pombe is essential for adaption to environmental changes in nutrient availability. ... The oxylipin NSF is a transmissible signal that activates mitochondrial respiration to maximize growth. ... [NSF/MeOH]) from two repetitive experiments (Exp.1 and Exp.2 ...